All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Ibrutinib for CLL: Mechanism of action and clinical considerations
Do you know... Which of the following pathways does ibrutinib primarily disrupt in CLL cells?
Chronic lymphocytic leukemia (CLL) is one of the most common forms of leukemia that affects adults.1 Previously, chemoimmunotherapy was the standard treatment approach for patients with CLL; however, with a median age at diagnosis of 72 years, many patients have comorbidities that preclude them from intensive therapies.1-3 Additionally, chemoimmunotherapy may not be suitable for patients with high-risk features, such as del(17p) or TP53 mutations.4Over the last decade, the treatment landscape for CLL has significantly changed with the advent of novel targeted therapies. This includes the introduction of Bruton’s tyrosine kinase (BTK) inhibitors, such as ibrutinib.1
Ibrutinib is a first-in-class, oral, irreversible, covalent inhibitor of BTK, which was first approved for patients with CLL in the US in 2013 and is currently indicated for first-line (1L) and second-line (2L) treatment of patients with CLL with or without del(17p).1,4 Below, we discuss the mechanism of action of ibrutinib and clinical considerations for its use in patients with CLL.
Mechanism of action
The B-cell receptor (BCR) signaling pathway is essential for B-cell proliferation, differentiation, and apoptosis.5 BTK is a key enzyme in this pathway.3 When the BCR is activated by antigen binding, protein tyrosine kinases activate BTK, which then activates phosphatidylinositol 3-kinase (PI3K), converting phosphatidylinositol 4,5‑bisphosphate (PIP2) into phosphatidylinositol 3,4,5‑trisphosphate (PIP3).5 This process promotes intracellular calcium release and triggers key signaling pathways that promote cell proliferation while inhibiting apoptosis.5
Ibrutinib covalently and irreversibly binds to cysteine 481 (C481) in the adenosine triphosphate (ATP)-binding site in the kinase domain of BTK (Figure 1).2,3 This inhibits the phosphorylation of downstream kinases in the BCR signaling pathway, subsequently inhibiting cell growth, migration, proliferation, and survival of malignant B cells.3,5 Inhibition of BTK has been shown to reduce pro-inflammatory cytokines and chemokines for homing to lymph nodes (Figure 1).5Ibrutinib can bind to interleukin (IL)-2-inducible kinase (ITK) in T cells, enhancing tumor immune surveillance.5 Ibrutinib also targets the CXCR4/SDF1 axis, downregulating CD20 expression on B cells.5 Finally, BCR signaling blockade through BTK inhibition with ibrutinib may indirectly affect the tumor microenvironment by promoting a pro-apoptotic environment.2
The activity of ibrutinib is complementary to venetoclax, a B-cell lymphoma-2 (BCL-2) inhibitor, allowing for a synergistic treatment combination (Figure 1).6 Ibrutinib reduces cell surface levels of CXCR4, resulting in CLL cells being released from the spleen and lymph node into the peripheral blood. Ibrutinib also indirectly decreases MCL-1 protein levels, leaving these CLL cells highly dependent on BCL-2 signaling.6 Venetoclax directly targets BCL-2, resulting in CLL cell apoptosis.6 In addition, BTK mutations may prevent the binding of ibrutinib, a key mechanism of resistance, further emphasizing the complementary proapoptotic effects of venetoclax.6 Finally, ibrutinib and venetoclax selectively act on different CLL subpopulations, with the proliferation subset responding more favorably to ibrutinib, and the quiescent subpopulation responding more to venetoclax.6 The comprehensive approach of combining both therapies, and the nonoverlapping toxicity profiles, suggests promising therapeutic synergy between the two agents.6
Figure 1. Ibrutinib mechanism of action and synergistic effects when combined with venetoclax*
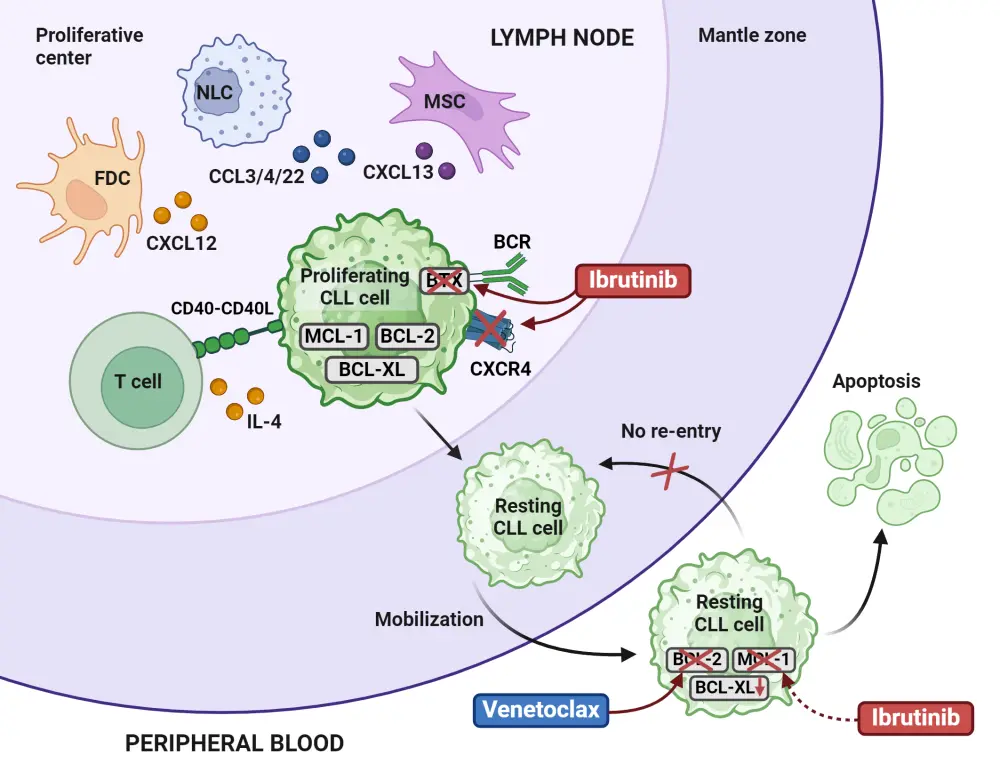
BCR, B-cell receptor; CLL, chronic lymphocytic leukemia; CCL3/4/22, C-C motif chemokine ligands 3, 4, and 22; CXCL12/13, chemokine C-X-C motif ligands 12 and 13; CXCR4, C-X-C chemokine receptor type; FDC, follicular dendric cell; MSC, mesenchymal stem cell; NLC, nurse-like cell.
*Adapted from Timofeeva, et al.6 Created with BioRender.com.
Clinical considerations
Several studies have demonstrated the benefit of ibrutinib, either alone or in combination with other therapies versus standard of care, in patients with treatment-naïve and relapsed/refractory (R/R) CLL (Table 1 and Table 2, respectively).
Table 1. Phase III trials of ibrutinib in treatment-naïve patients with CLL*
|
Study |
Treatment |
Patients, n |
Median follow-up, months |
ORR (CR) |
Median PFS, months |
Median OS, months |
|
Ibrutinib vs chlorambucil |
136 vs 133 |
18.4 |
86% (4%) vs 35% (2%) |
NR vs 18.9 (p < 0.001) |
HR, 0.16; 95% CI, 0.05–0.56; p = 0.001 |
|
|
Ibrutinib + obinutuzumab vs chlorambucil + obinutuzumab |
113 vs 116 |
45 |
91% (40%) vs 81% (16%) |
NR vs 22 (p < 0.0001) |
NR vs NR (p = 0.793) |
|
|
BR vs ibrutinib vs ibrutinib + rituximab |
183 vs 182 vs 182 |
55 |
— |
44 vs NR vs NR (p < 0.0001) |
84% vs 85% vs 86% at 48 months |
|
|
GLOW10 (NCT03462719) |
Ibrutinib + venetoclax vs chlorambucil + obinutuzumab |
106 vs 105 |
46 |
43% vs 12%* |
NR vs 21.7 (p < 0.0001) |
HR, 0.487; 87.5% vs 77.6% at 42 months |
|
FLAIR11(ISRCTN01844152) |
Ibrutinib + rituximab vs FCR |
386 vs 385 |
53 |
91% (21%) vs 88% (61%) |
NR vs 67 months (p < 0.0001) |
NR vs NR (p = 0.96) |
|
BR, bendamustine + rituximab; CI, confidence interval; CLL, chronic lymphocytic leukemia; CR, complete response; FCR, fludarabine + cyclophosphamide + rituximab; HR, hazard ratio; NR, not reached; ORR, overall response rate; OS, overall survival; PFS, progression-free survival. *CR/CR with incomplete count recovery. |
||||||
Table 2. Phase III trials of ibrutinib in patients with R/R CLL*
|
Study |
Treatment |
Patients, n |
Median follow-up, months |
ORR (CR) |
Median PFS, months |
Median OS, months |
|
Ibrutinib vs ofatumumab |
195 vs 196 |
65.3 |
91% (11%) for ibrutinib |
44.1 vs 8.1 (p < 0.0001) |
67.7 vs 65.1 (HR, 0.810) |
|
|
Ibrutinib + BR vs BR |
289 vs 289 |
34.8 |
87.2% (38.1%) vs 66.4% (8.0%) |
NR vs 13.3 (p < 0.0001) |
NR vs NR (p = 0.019) |
|
|
Ibrutinib+ ublituximab vs ibrutinib |
64 vs 62 |
41.6 |
83% (17%) vs 65% (3%) |
NR vs 35.9 (p = 0.016) |
NR vs NR (p = 0.12) |
|
|
BR, bendamustine and rituximab; CR, complete response; HR, hazard ratio; NR, not reached; ORR, overall response rate; OS, overall survival; PFS, progression-free survival. |
||||||
Patient considerations
Second-generation BTK inhibitors were designed to be more specific to the BTK enzyme, resulting in less off-target activity.2 Head-to-head trials have demonstrated non-inferior clinical efficacy of second-generation BTK inhibitors vs ibrutinib monotherapy, with improved toxicity profiles.2 As such, when deciding between ibrutinib and second-generation BTK inhibitors, patient comorbidities, age and fitness, efficacy, expected safety profile, and cost should be considered.2 The European Society for Medical Oncology (ESMO) interim update to their clinical practice guidelines recommends second-generation BTK inhibitors over ibrutinib in patients with an increased risk of cardiac toxicity.15 Conversely, in patients with a history of migraine or chronic cough, or who require long-term proton pump inhibitor treatment, ibrutinib may be the preferred option.2 Additionally, ibrutinib can be effectively combined with venetoclax, improving efficacy.6,15
Patients with CLL with high-risk features such as mutated TP53 or del(17p) historically do not respond well to chemoimmunotherapy.2 Results from the phase II CAPTIVATE trial (NCT02910583) demonstrated the benefit of ibrutinib plus venetoclax in patients with CLL with high-risk genomic features.16 The overall response rates (ORRs) were ≥95% irrespective of high-risk features.16 Additionally, the 36-month progression-free survival (PFS) and overall survival (OS) rates were similar between patients with or without high-risk features (Figure 2).16 These findings were confirmed in the 5.5-year follow-up of this trial.
Figure 2. 36-month PFS and OS in the phase II CAPTIVATE trial*
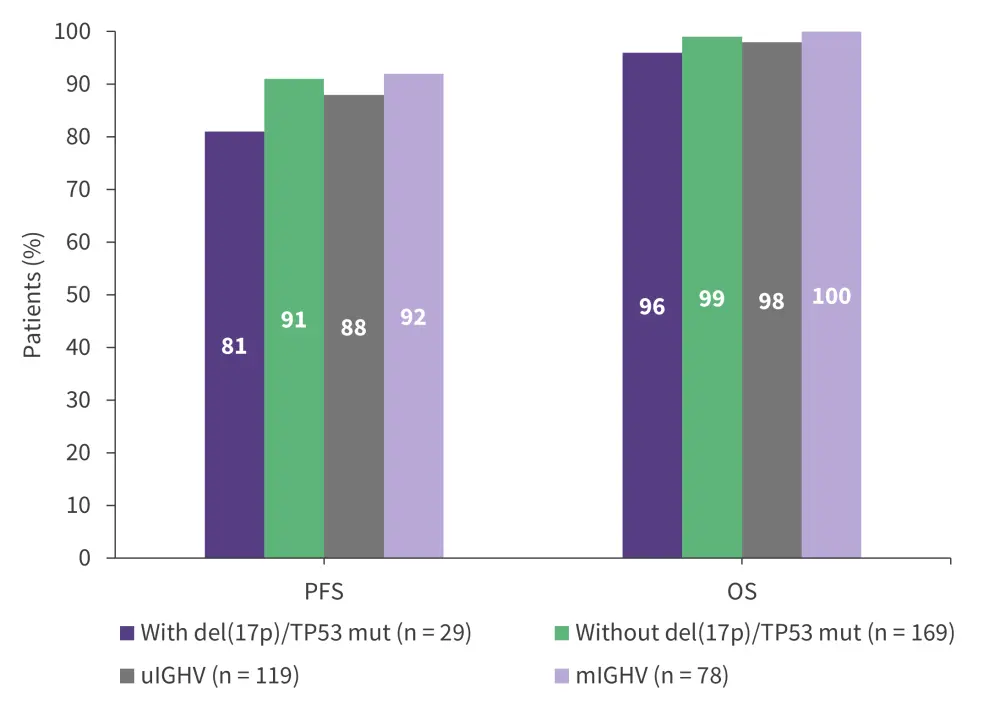
mIGHV, mutated IGHV; OS, overall survival; PFS, progression-free survival; TP53mut, mutated TP53; uIGHV, unmutated IGHV.
*Data from Allan, et al.16
Results from the 4-year update of the phase III GLOW trial demonstrated that fixed-duration ibrutinib plus venetoclax improved PFS (hazard ratio [HR], 0.214; 95% confidence interval [CI], 0.138–0.334; p <0.0001) and OS (HR, 0.487; 95% CI, 0.262–0.907; p = 0.021) vs chlorambucil plus obinutuzumab in older patients with CLL.10 Based on results from this trial and results from the time-limited cohort in the CAPTIVATE trial, time-limited ibrutinib plus venetoclax for 12 cycles has been approved by the European Medicines Agency (EMA) for patients with treatment-naive CLL.15 The ESMO clinical practice guidelines recommend that tolerability and reimbursement for prolonged treatment be considered when deciding between fixed-duration ibrutinib or personalized treatment based on measurable residual disease.15 During the Lymphoma Hub Hub Steering Committee meeting, Astrid Pavlovsky, Fundaleu, Buenos Aires, AR, chaired a discussion on the phase III GLOW trial: ibrutinib plus venetoclax in previously untreated CLL/small lymphocytic lymphoma.
Phase III GLOW trial: Ibrutinib plus venetoclax in previously untreated CLL/SLL
In the phase III ALLIANCE A041202 trial, while the protective effects of ibrutinib-based treatment were observed across subgroups, ibrutinib was even more protective in patients with TP53 abnormalities (HR, 0.07; 95% CI, 0.03–0.18) or ZAP-70 unmethylated disease, used as a surrogate for unmutated IGHV (HR, 0.18; 95% CI, 0.12–0.28).9 Based on these results, ibrutinib is favored over chemoimmunotherapy in patients with high-risk CLL.
Drug interactions
Ibrutinib, metabolized by the liver, is a major substrate of CYP3A4 and a minor substrate of CYP2D6.5 Concomitant use of strong CYP3A4 inhibitors or inducers should be avoided.5 If ibrutinib is given with voriconazole or posaconazole, the ibrutinib dose should be reduced.5
Preventing and managing toxicities
Cardiovascular toxicities such as arrhythmia, particularly atrial fibrillation, and hypertension, have been associated with BTK inhibitors.2 While arterial fibrillation is more common with ibrutinib, it can also occur with second-generation BTK inhibitors. In rare cases, patients have experienced ventricular arrhythmia and congestive heart failure. A baseline electrocardiogram and echocardiogram should be obtained prior to treatment initiation. Other risk factors for atrial fibrillation such as age, hypertension, and prior exposure to cardiotoxic chemotherapy should be considered in clinical decision-making.2
A pooled analysis of 10 studies of ibrutinib treatment in patients with CLL (n =781), mantle cell lymphoma (n = 250), marginal zone lymphoma (n = 63), and Waldenstrom’s macroglobulinemia (n = 169) assessed the impact of dose reductions due to cardiac adverse events (AEs).17 in total, 19% of patients experienced cardiac AEs of any grade. Patients with dose reductions had less recurrence of the same cardiac AE at the same or worse severity compared with patients with no dose reduction overall (14% vs 18%) and as serious AEs (5% vs 10%). Among patients who started at the 420 mg dose of ibrutinib, dose interruptions effectively reduced the rate of cardiac AEs recurring at the same or worse severity vs patients with no dose interruptions (0% vs 19%). Importantly, PFS was not impacted by dose reductions in all patients (24-month PFS rate, 91%) and in patients who started at the 420 mg dose (24-month PFS rate, 94%).17
Bleeding is another common toxicity of covalent BTK inhibitors, and a comprehensive bleeding risk assessment should be performed before therapy initiation.2 Low-grade bleeding can be managed with supportive care, while high-grade bleeding may require hospitalization and blood and platelet transfusions. Other common toxicities include infection, cytopenia, and diarrhea. Psuedo-Richter transformation can occur when treatment with BTK inhibitors is paused. Resumption of BTK inhibition has resulted in the regression of transformation in some cases, and this should be attempted prior to conventional intensive chemotherapy.2
Clinical insights from real-world practice
Prospective, real-world studies offer valuable insights to complement the data from clinical trials of ibrutinib in CLL. The EVIdeNCE and REALITY studies confirm the efficacy and safety profile of ibrutinib in patients with CLL within routine clinical practice.18 Additionally, these studies demonstrate the impact of different therapeutic lines on retention rates.18
EVIdeNCE19
The prospective, multicenter, observational EVIdeNCE study (NCT03720561) assessed ibrutinib utilization patterns in real-world settings in Italy. In total, 309 patients were included, of which 38.2% initiated 1L ibrutinib treatment, 41.1% were in the 2L, and 20.7% were in the third or more line (≥3L). The median follow-up was 23.9 months, and 229 patients completed the 24-month observational period.
The overall retention rate for 2 years was 70.2%, and the retention rate was higher among patients who started ibrutinib in the 1L vs 2L or ≥3L (Figure 3).
Figure 3. 1- and 2-year ibrutinib retention rates in the EVIdeNCE study*
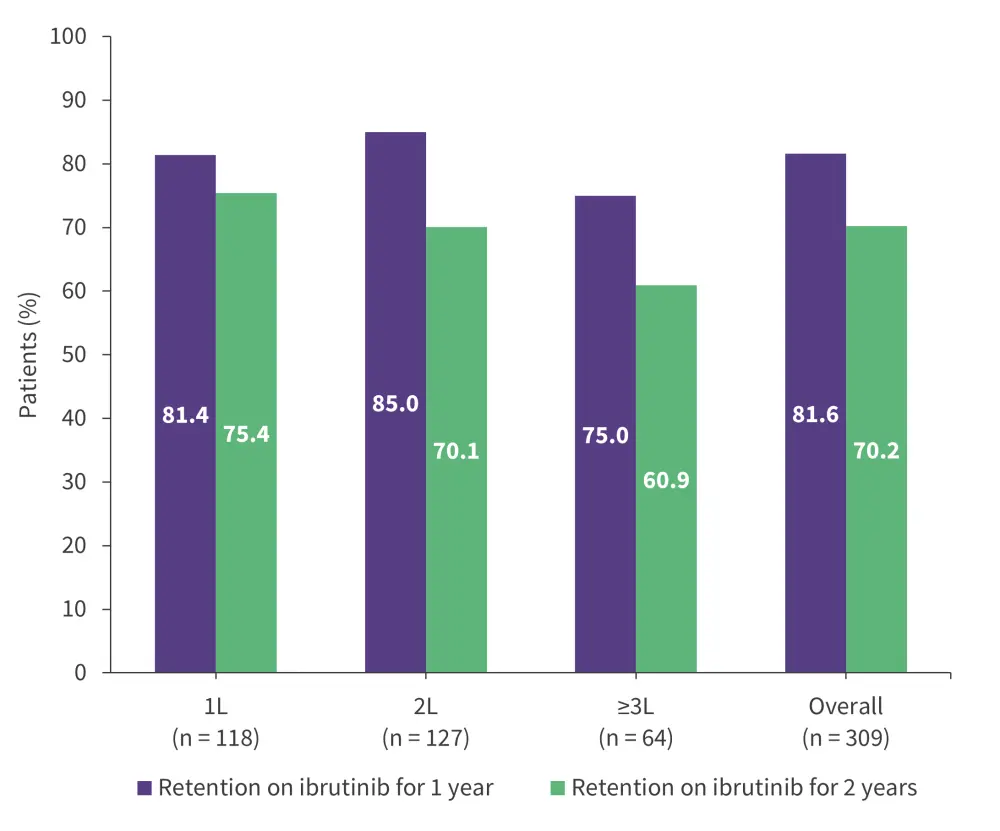
1L, first-line; 2L, second-line; 3L, third-line.
*Adapted from Mauro, et al.19
- 34.6% of patients had a temporary dose interruption, with a median duration of 2 weeks, most commonly due to AEs.
- 29.8% of patients permanently discontinued treatment, mostly due to AEs (14.2%), death (5.8%), and progressive disease (4.9%).
- The ORR and complete response (CR) rate was 75.9% and 18.4%, respectively (Figure 4).
Figure 4. Response rates with ibrutinib observed in the EVIdeNCE study*
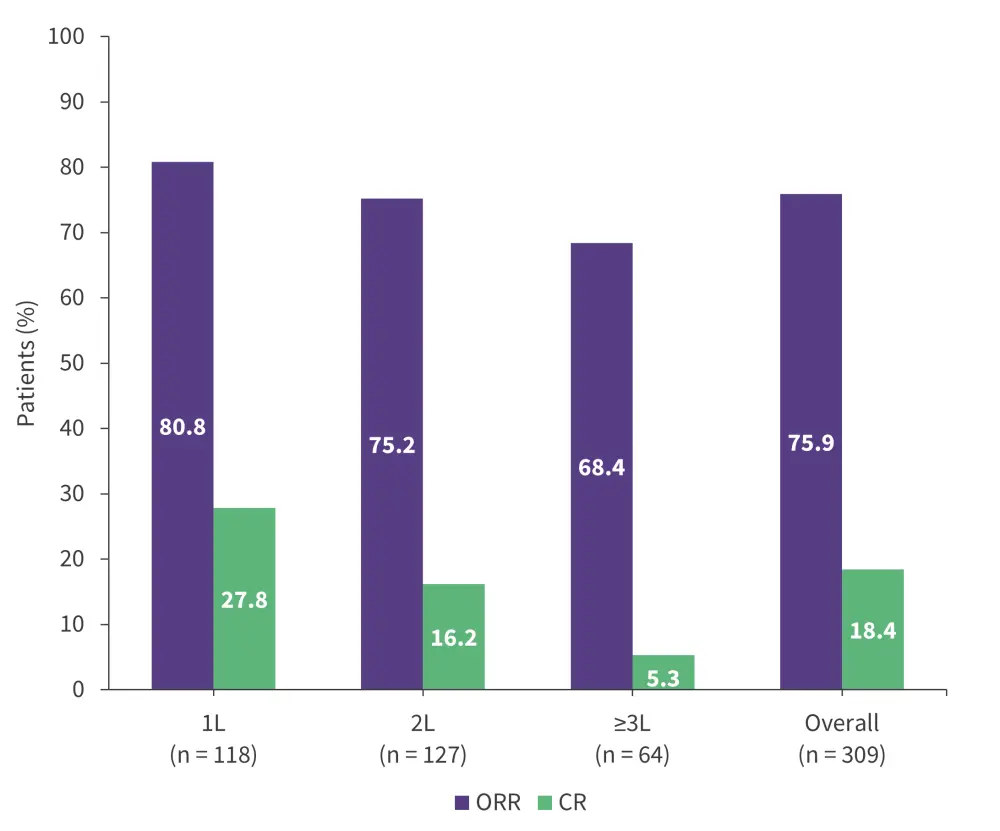
1L, first-line; 2L, second-line; 3L, third-line; CR, complete response; ORR, overall response rate.
*Data from Mauro, et al.19
Median PFS was not reached, and 2-year survival outcomes are shown in Figure 5.
Figure 5. 2-year PFS and OS rates with ibrutinib in the EVIdeNCE study*
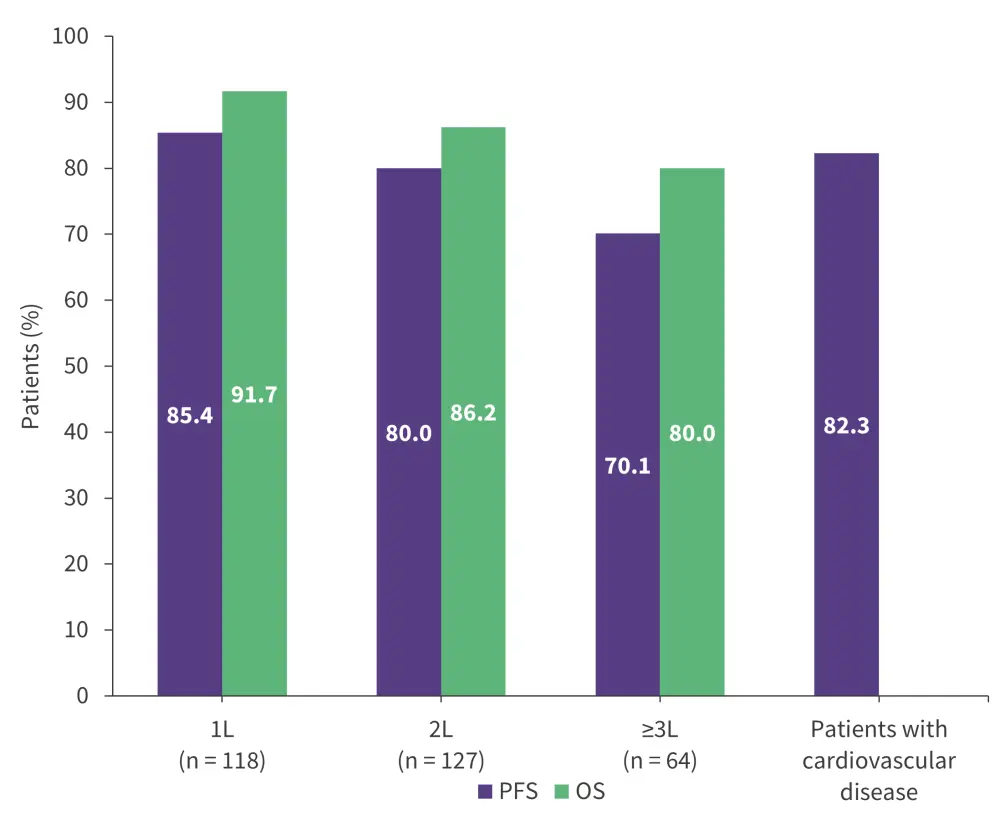
1L, first-line; 2L, second-line; 3L, third-line; OS, overall survival; PFS, progression-free survival.
*Data from Mauro, et al.19
- Any grade and Grade 3–4 AEs occurred in 75.4% and 34.6% of patients, respectively, and the safety profile was consistent with those observed in clinical trials.
- 41 patients died; 19 due to infections, 9 due to disease progression, 5 due to secondary malignancies, and 3 due to cardiovascular events.
REALITY20
The prospective, multicenter, observational REALITY study assessed the retention rate and clinical outcomes of patients with CLL treated with ibrutinib in real-world settings in Germany. In total, 302 patients were included (1L, n = 104; 2L, n = 90; ≥3L, n = 108). Patient typology was assessed in 280 patients and categorized based on the patient's level of acceptance (feeling that their health status is acceptable) and control (belief that their health status can be controlled by themselves or others). The median follow-up was 30.9 months.
The overall retention rate for 1 year was 69.9%, with lower retention rates for patients who received ibrutinib in the ≥3L vs 1L (p = 0.003; Figure 6).
Figure 6. 1-year retention rates with ibrutinib by treatment line in the REALITY study*
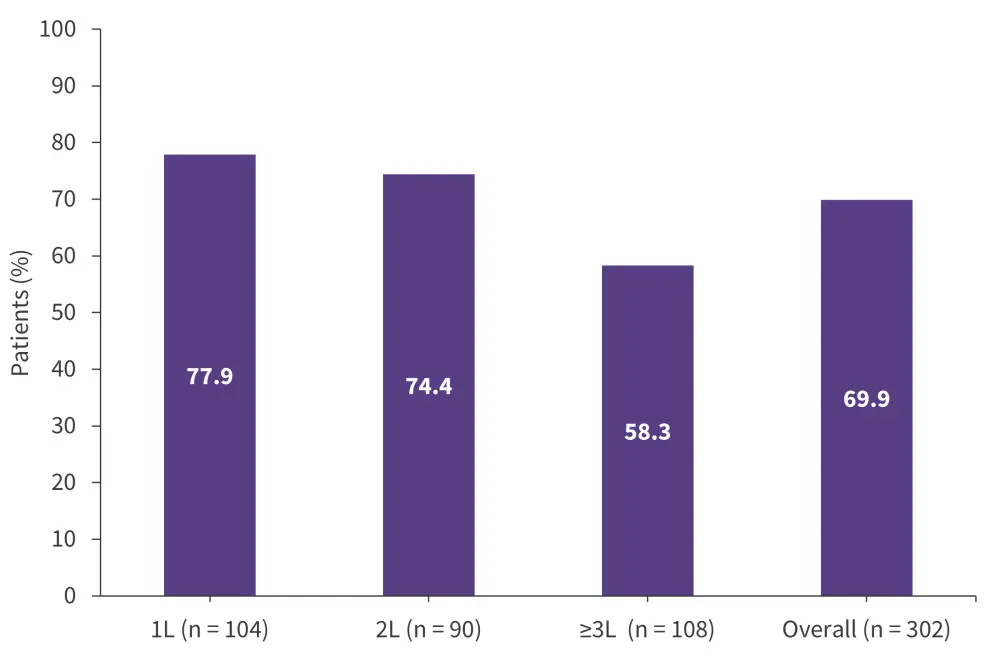
1L, first-line; 2L, second-line; 3L, third-line.
*Adapted from Gerhardt, et al.20
Patients with high acceptance and either high control or low control were more likely to remain on treatment than patients with low acceptance (Figure 7).
Figure 7. 1-year retention rates by patient typology in the REALITY study*
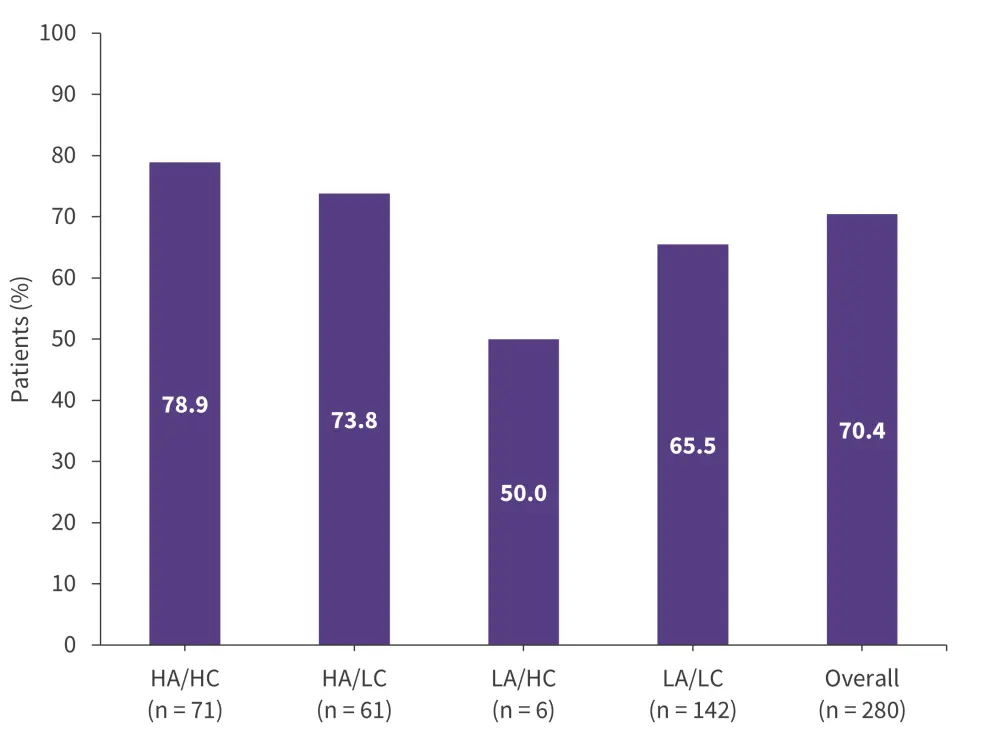
HA, high acceptance; HC, high control; LA, low acceptance; LC, low control.
*Adapted from Gerhardt, et al.20
- In total, 50.3% and 33.8% discontinued treatment permanently or temporarily, respectively.
- The main reasons for permanent discontinuation were Grade ≥3 AEs (48.7%), Grade 1–2 AEs (17.1%), disease progression (11.8%), other concomitant medication (3.3%), stem cell transplantation (1.3%), or surgery (0.7%).
- Efficacy outcomes were consistent with existing clinical trial data, confirming the effectiveness of ibrutinib in real-world patients.
- The ORR and CR rate was 80.8% and 14.2%, respectively.
- 2-year PFS and OS rates were 77.8% and 90.7%, respectively.
- AEs and treatment-related AEs occurred in 73.2% and 39.4% of patients, respectively, and the safety profile was similar to those reported in clinical trials.
- 23 patients died, including 2 patients who died due to cardiac disorders.
Conclusion
Ibrutinib is an important treatment option for both treatment-naïve and R/R patients with CLL. Identifying patients who are most likely to benefit from ibrutinib and consideration of patient-related factors, such as comorbidities and high-risk features, are crucial to optimizing outcomes.2 The monitoring and management of AEs play a vital role in the safe long-term use of ibrutinib.2 Despite these challenges, ibrutinib maintains a pivotal role in the treatment of patients with CLL.18The synergistic effect of combining ibrutinib with venetoclax has resulted in improved outcomes for CLL, and several future trials may explore novel combinations.6 Integrating real-world data, such as the results from the EVIdeNCE and REALITY studies, along with proactive patient management, can continue to enhance patient outcomes.18 Further studies may advance ibrutinib treatment, with a shift towards a more personalized treatment approach.18
Your opinion matters
As a result of this content, I commit to reviewing the clinical considerations for ibrutinib to guide my treatment of patients with CLL in clinical practice.
This educational resource is independently supported by Pharmacyclics LLC, an AbbVie Company, and Janssen Biotech, Inc., administered by Janssen Scientific Affairs, LLC. All content is developed by SES in collaboration with an expert steering committee; funders are allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content




