All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Do you know... What major changes have been made to the Austrian CAR T-cell Network algorithm from 2022 compared with 2019?
Video series
Here, we share a presentation by Ulrich Jӓger, Medical University of Vienna, Vienna, AT, on a case study to evaluate sequencing therapies for patients with high-risk diffuse large B cell lymphoma (DLBCL).
The Lymphoma Hub has previously covered sequencing therapies for patients with high risk DLBCL.
In this presentation, Jӓger shares a case study from 2019 of a 59-year-old female patient with DLBCL and the treatment decisions leading to chimeric antigen receptor (CAR) T-cell therapy, changes from 2019 to 2022 onwards in the algorithm for patients with DLBCL in clinical routine (Figure 1), and results in relapsed/refractory (R/R) patients with low-risk DLBCL treated with CAR T-cell therapy, presented at the European Hematology Association (EHA) 2023 Hydrid Congress (Figure 2).
Figure 1. Selection algorithm for patients with DLBCL in clinical routine: Austrian CAR T-cell Network*
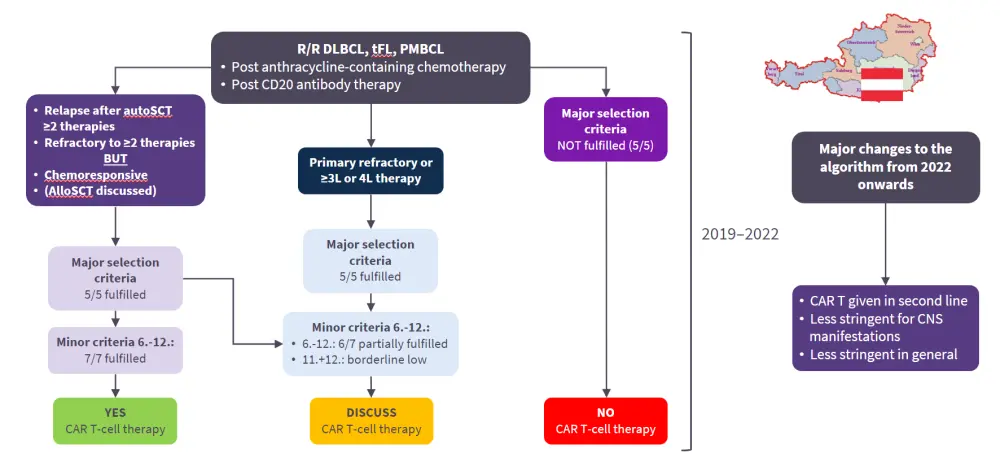
*Adapted from Greinix, et al.1
Figure 2. Probability of OS and PFS of patients with R/R LBCL infused with commercial CAR T-cell compounds*
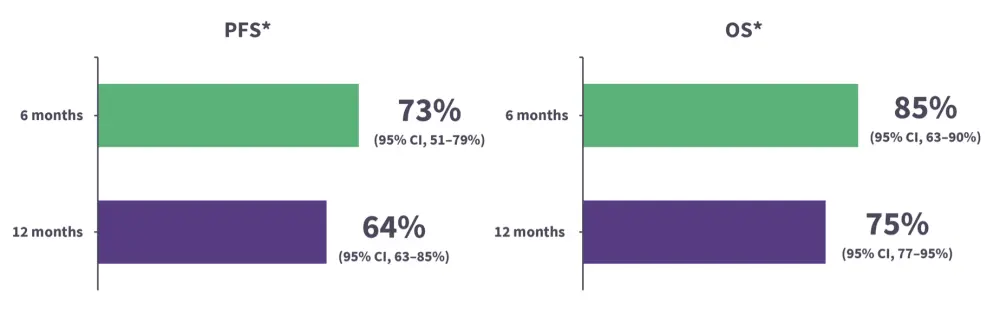
CAR, chimeric antigen receptor; CI, confidence interval; LBCL, large B-cell lymphoma; OS, overall survival; PFS, progression-free survival; R/R, relapsed/refractory
*Adapted from Rudzki, et al.2
†Data from 65 patients treated with axicabtagene ciloleucel or tisagenlecleucel.
Watch or download the presentation to learn more about the understanding of CAR T-cell therapy in patients with DLBCL, including:
- A real-world evidence case study of a patient with DLBCL treated in 2019 according to the algorithm for patients with DLBCL in clinical routine.
- The key changes to the algorithm from 2019 to 2022 onwards, focusing on the removal of the requirement of no central nervous system (CNS) involvement in their diagnosis.
- Data presented from the 64th American Society of Hematology (ASH) Annual Meeting and Exposition on progression-free survival and overall survival in low-risk patients with DLBCL treated with CAR T-cell therapy.
Key points
- Special populations, such as patients with secondary CNS lymphoma, can be included in CAR T-cell treatments.
- Secondary CNS lymphoma can be successfully treated with CAR T-cell therapy.
- Sequencing capabilities have rapidly advanced since 2019 and earlier CAR T-cell therapy use in first-line high-risk DLBCL is recommended, while use in second line is a standard-of-care treatment for some lymphoma subtypes.
- CAR T-cell therapy is preferred to autologous stem cell transplant and complete response is no longer required.
This activity was supported through an educational grant from Bristol Myers Squibb.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Ulrich Jäger
Ulrich Jäger


