All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Genomic pathways and treatment strategies for patients with Waldenstrom’s macroglobulinemia
Do you know... Resistance to which of the following therapies used to treat Waldenstrom’s macroglobulinemia has been associated with mutated CXCR4?
Introduction
Waldenstrom’s macroglobulinemia (WM) is a rare type of B-cell malignant lymphoma, clinically presented as infiltration of lymphoplasmacytic cells and production of monoclonal IgM proteins. Despite its rarity, there has been vast progression of WM treatments in both frontline and relapsed/refractory (R/R) settings.1 Advancements in high-technology sequencing have led to the elucidation of an evolving molecular landscape, which has become increasingly important for disease management in WM.2
Treatments involving Bruton’s tyrosine kinase inhibitors (BTKis), which are based on the integral role of BTK as a signaling molecule in the pro-survival of WM cells, have paved the way for the successful treatment of patients with WM.1 In this article, we summarize the genomic landscape of WM, current and emerging BTKi therapies, and the implications for treatment options and outcomes in patients with WM, as presented at the recent 2022 IWMF Educational Forum.
Genomics and BTK signaling pathway in WM
First identified in next-generation sequencing studies, the myeloid differentiation primary response 88 (MYD88) L265P is the most common mutation in patients with WM, occurring in 95–97% of cases. A few pathways have been described for mutated MYD88. One such pathway is the dimerization of MYD88 mutated cells upon toll-like receptor activation which induces assembly of a myddosome complex with interleukin-1 associated kinases (IRAK), IRAK1/IRAK4 and BTK. The downstream signaling of this complex trigger activation of nuclear factor kappa B, involved in proliferation and survival of WM cells (Figure 1).3
Another pro-survival mutated MYD88 signaling pathway involves activation of hematopoietic cell kinase proteins via interleukin-6 receptors, which can trigger a plethora of downstream pathways including BTK, phosphoinositide-3-kinase–protein/AK strain transforming or protein kinase B (AKT), and mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK)1/2. Recently, mutated MYD88 has been shown to associate with the B-cell receptor pro-survival signaling pathway via the spleen tyrosine kinase protein, which activates the signal transducer and activator of transcription 3 and AKT proteins (Figure 1).3
Figure 1. Mutated MYD88 pro-survival signaling pathways in WM*
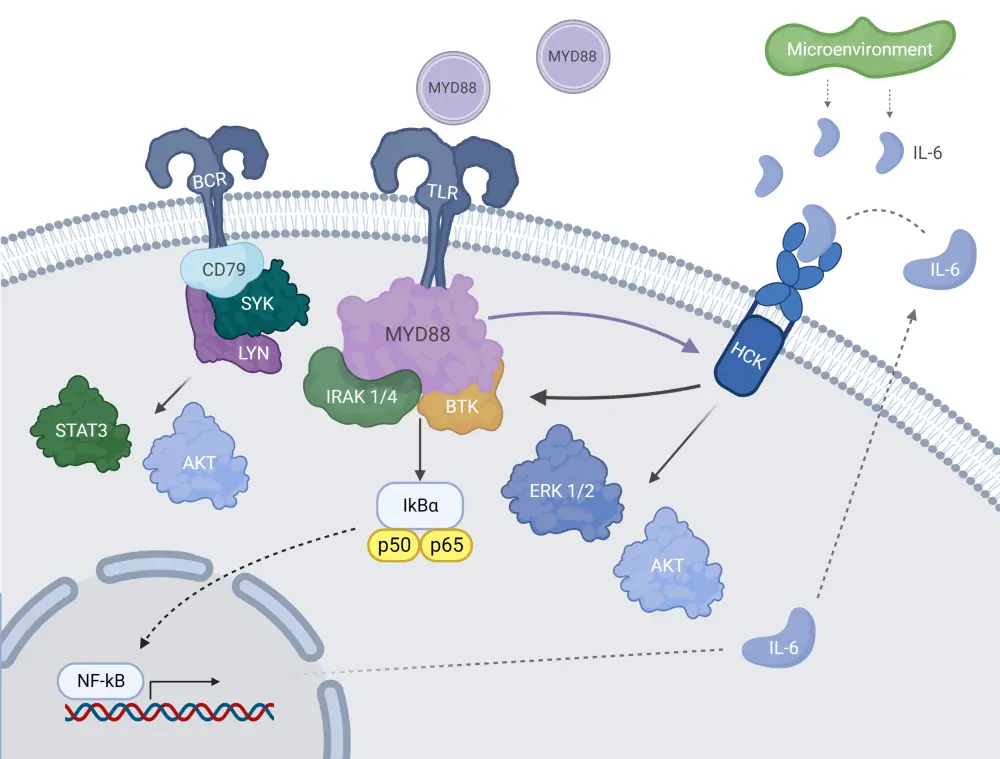
AKT, Ak strain transforming/protein kinase B; BCR, B-cell receptor; BTK, Bruton’s tyrosine kinase; ERK 1/2, extracellular signal-regulated kinase 1/2; HCK, hematopoietic cell kinase; IL-6, interleukin-6; IRAK 1/4, interleukin-1 associated kinases 1/4; LYN, Lck/Yes novel tyrosine kinase ; MYD88, myeloid differentiation primary response 88; NF-kB, nuclear factor kappa B; STAT3, signal transducer and activator of transcription 3; SYK, spleen tyrosine kinase protein; TLR, toll-like receptor; WM, Waldenstrom’s macroglobulinemia.
*Adapted from Treon, et al.3 Created with BioRender.com
The second most common mutation, accounting for up to 40% of WM cases, is the C-X-C chemokine receptor type 4 (CXCR4). Unlike MYD88, CXCR4 mutation is highly variable and has both nonsense and frameshift variants, but the WHIM (warts, hypogammaglobulinemia, infection, and myelokathexis) sub-type so far is the most identified. This pathway involves internalization of the receptor once mutated CXCR4 and its associated ligand, C-X-C Motif Chemokine Ligand 12, binds to it; downstream signaling of the pro-survival AKT and ERK pathways then occurs.4
BTK inhibitors in the frontline and R/R setting for WM
Ibrutinib, an oral BTKi, is the first to receive U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) approval for the treatment of patients with WM. FDA approval in 2015 was based on high response rates in the primary analysis of a phase II study (NCT01614821) involving patients with relapsed/refractory (R/R) WM (n = 63).5,6 An overall response rate (ORR) of 91% and major response rate (MRR) of 73% was reported. The longer-term follow-up of ibrutinib in this population subset demonstrated its durable efficacy, with a consistent ORR and a higher MRR reported (Table 1).7 In a sub-study analysis of patients (n = 31) with rituximab-refractory WM, ibrutinib achieved a good ORR and MRR overall (Table 1).8
In the frontline setting, ibrutinib has demonstrated high efficacy in a primary analysis of previously untreated patients, with an ORR and MRR of 100% and 83% respectively.9 Similar efficacy was seen in the follow-up study.10
Despite the success of ibrutinib, concerns around associated toxicities and acquired resistance have led to the development of next-generation inhibitors such as zanubrutinib and acalabrutinib.1 Zanubrutinib is a second-generation BTKi which was proven to be highly effective in a phase I/II 3-year follow-up study for both treatment-naïve (n = 24) and R/R (n = 53) patients with WM, achieving an ORR of 95.9%, MRR of 82.2%, very good partial response (VGPR) rate of 45.2%, and acceptable safety profile across both cohorts.1,11 At the 3-year mark, the estimated progression-free survival (PFS) and overall survival was 80.5% and 84.8%, respectively.
Zanubrutinib was the second BTKi FDA-approved in 2021,12 following the high efficacy and safety seen in the phase III ASPEN trial of ibrutinib vs zanubrutinib; the results of which are shown in Table 1.13 Although response rates between the two were comparable, zanubrutinib did achieve a higher VGPR (28% vs 19%) and reported fewer adverse events overall compared with ibrutinib, demonstrating its potential as a safer treatment option. Acalabrutinib is another next-generation inhibitor which was trialed in a cohort of both treatment-naïve (n = 14) and R/R (n = 92) patients with WM.14
A novel BTKi on the horizon is pirtobrutinib, a selective and reversible BTKi shown to block BTK and ERK activation in mutated MYD88 cells expressing the BTKC481S mutation. In the BRUIN trial, previously reported on the Lymphoma Hub, pirtobrutinib achieved an ORR of 69% in patients with WM. Table 1 outlines the efficacy of acalabrutinib, ibrutinib, and zanubrutinib in treatment naïve and R/R patients with WM.
Table 1. Summary of selected clinical studies for frontline and R/R therapy in patients with WM*
|
MRR, major response rate; ORR, overall response rate; PFS, progression-free survival; R/R, relapsed/refractory; TN, treatment-naïve; WM, Waldenstrom’s macroglobulinemia. |
||||
|
Study regimen, % (unless otherwise stated) |
N |
ORR |
MRR |
PFS |
|---|---|---|---|---|
|
Ibrutinib in R/R patients |
63 |
91% |
79% |
54% at 5 year |
|
Ibrutinib in rituximab-refractory patients |
31 |
87% |
71% at 18 months |
Median of 39 months |
|
Ibrutinib in TN patients |
30 |
100% |
87% |
76% at 4 years |
|
Zanubrutinib in TN + R/R patients (ASPEN) |
101 |
94% |
77% |
85% at 18 months |
|
Acalabrutinib in TN + R/R patients |
106 |
93% |
80% |
80–90% at 2 years |
Role of genomics in treatment decision making
Impact of genomic landscape on treatment outcomes
MYD88 and CXCR4 mutational status in WM has demonstrated different clinical presentations and sensitivities to BTKis given their different signaling pathways. There is growing evidence of the impact of mutational status on the depth and timing of response and PFS, with the CXCR4 mutation being associated with inferior treatment outcomes overall.3
In the primary analysis of ibrutinib in patients with R/R WM,6 treatment outcomes by mutational status revealed that there was a higher ORR and MRR amongst those with the MYD88mut/CXCR4wt than those with MYD88mut/CXCR4mut and MYD88wt/CXCR4wt genotypes. The ORR was 100%, 85.7%, and 71.4%, respectively, and the MRR was 91.2%, 61.9%, and 28.6%, respectively.
The follow-up analysis of ibrutinib in patients with WM who had R/R disease showed a similar pattern of response rates by mutational status.7 Statistical analysis showed a higher significant difference in ORR (p < 0.01) and MRR (p < 0.0001) for MYD88mut CXCR4wt vs MYD88mut CXCR4mut genotypes. The VGPR rate was higher for MYD88mut than for CXCR4mut, and the median time to achieve a major response was delayed for those with MYD88mut/CXCR4mut compared with MYD88mut/CXCR4wt. The PFS was also shorter for those with CXCR4 mutated status. Response rates, timing of response, and PFS results can be seen in Table 2.
Results for treatment outcome by mutational status for patients with rituximab-refractory WM demonstrate that ORR was not influenced by the genetic subtype; however, those with the MYD88mut/CXCR4wt achieved a numerically higher VGPR and MRR, as well as a shorter median time to attain a major response than those with the MYD88mut/CXCR4mut (Table 2).
In a primary study on ibrutinib in patients previously untreated for WM,9 all patients displayed the MYD88mut subtype with differences in the CXCR4 status. No difference was seen in ORR (100%); however, the MRR (71% vs 94%) and VGPR (7 vs 31%) was lower for patients with CXCR4mut compared to those without. A difference was seen in the timing for both the major (0.9 vs 1.7 months) and minor (1.8 vs 7.3 months) responses—shorter for the MYD88mut/CXCR4wt compared with the MYD88mut/CXCR4mut genotype.
Similar trends in the depth and timing of response were seen in the longer-term follow-up results of ibrutinib in patients previously untreated for WM (Table 2).10 The variability in responses to ibrutinib seen for MYD88 and CXCR4 mutational status could be explained by the strong involvement of BTK signaling for the MYD88 pathway and the AKT/ERK mediated pathway for CXCR4, shown to promote resistance to ibrutinib in preliminary studies.6
For zanubrutinib in patients with R/R and previously untreated WM, the ORR and MRR were comparable between the MYD88mut/CXCR4wt and MYD88mutP/CXCR4mut genotypes; however, there was a lower VGPR/complete response rate (27% vs 59%) observed in those with CXCR4mut compared with CXCR4wt. 11
Table 2. Summary of response rates and PFS by mutational status in selected trials of ibrutinib*
|
MR, minor response; MRR, major response rate; mut, mutated; ORR, overall response rate; PFS, progression-free survival; R/R relapsed/refractory; TN, treatment-naïve; VGPR, very good partial response; WM, Waldenstrom’s macroglobulinemia; wt, wild-type. |
|||
|
Response, % (unless |
MYD88mut/CXCR4wt |
MYD88mut/CXCR4mut |
MYD88wt/CXCR4wt |
|---|---|---|---|
|
Ibrutinib monotherapy in patients with R/R WM |
|||
|
ORR |
100 |
86.4 |
50 |
|
MRR |
97.2 |
68.2 |
— |
|
VGPR |
47.2 |
9.1 |
— |
|
Median time to minor |
1.8 |
4.7 |
— |
|
PFS |
70 |
38 |
— |
|
Ibrutinib in patients with rituximab-refractory WM |
|||
|
ORR |
88 |
86 |
— |
|
MRR |
88 |
71 |
— |
|
VGPR |
41 |
14 |
— |
|
Median time to major |
1 |
4 |
— |
|
Ibrutinib in patients with TN WM |
|||
|
ORR |
100 |
100 |
— |
|
MRR |
94 |
78 |
— |
|
VGPR |
44 |
14 |
— |
|
Median time to minor |
0.9 |
1.7 |
— |
|
Median time to major |
1.8 |
7.3 |
— |
Genomic-based algorithms for WM
Given the impact of MYD88/CXCR4 mutational status on treatment outcomes, such genotype data could be beneficial in guiding treatment options for patients with WM. Genomic-based treatment algorithms for patients in frontline (Figure 2 and R/R (Figure 3) settings have been devised by Treon, et al.,3 with updated data presented at the 2022 IWMF Educational Forum.
For patients with a MYD88 mutation previously untreated for WM, BTKi monotherapies such as ibrutinib are prioritized (Figure 2) given their success in the frontline setting. Moreover, as studies in MYD88 have proven that there is little difference in efficacy between the use of chemoimmunotherapy and a BTKi alone, single-agent ibrutinib can be used—avoiding the risk-associated toxicities of chemoimmunotherapy. Bendamustine plus rituximab (benda-R) or proteasome inhibitor (PI) based regimens are alternatives that can be considered for those with MYD88mut status; the former is especially important for patients presenting with bulky or extramedullary disease, and the latter for patients with symptomatic amyloidosis.3
For patients who are untreated and double mutated (MYD88mut/CXCR4mut), the key treatment consideration is whether a rapid response is required (Figure 2). If so, similar to patients with high IgM levels or symptomatic hyperviscosity, benda-R or a PI-based regimen can be considered. A BTKi may not be the best option in this case due to the time taken to achieve a response. In patients where a response is not urgently required, ibrutinib plus rituximab can be considered vs ibrutinib alone due to the improved responses demonstrated with combination treatment. Zanubrutinib at this stage is also a viable option.3
For patients with wild-type MYD88 and CXCR4, benda-R or a PI-based regimen can be used as studies have shown them to be efficacious in this population subset. Zanubrutinib is another good option for this genotype subset after a substudy of the ASPEN trial showed a clinically meaningful response in the MYD88 wild-type WM cohort, as previously reported on the Lymphoma Hub.3
Figure 2. Genomic-based treatment approach for previously untreated patients with WM*
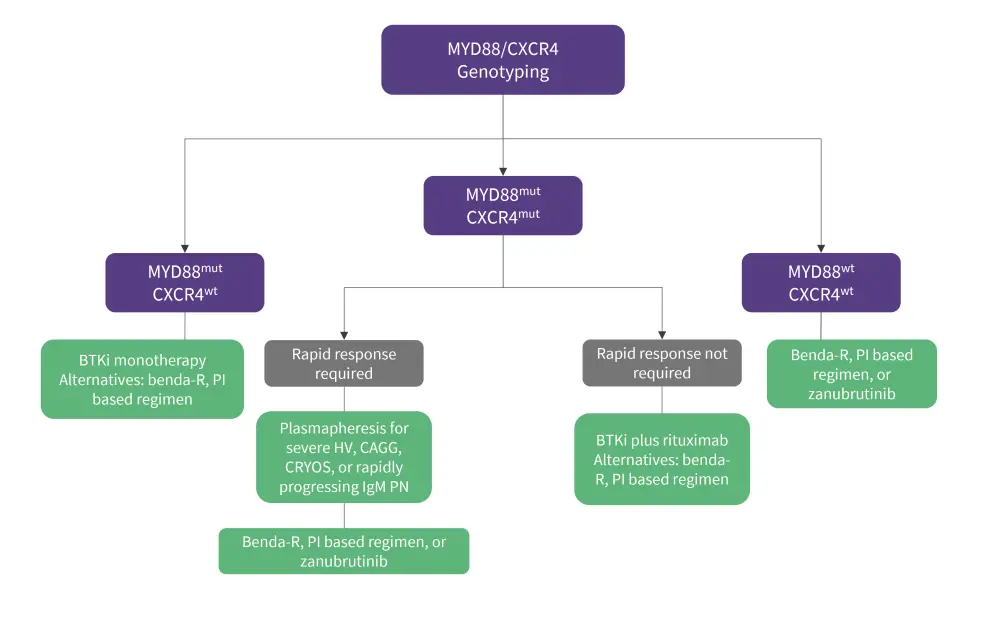
benda-R, bendamustine plus rituximab; BTKi, Bruton’s tyrosine kinase inhibitors; CAGG, cold agglutinemia; CRYOS, cryoglobulinemia; HV, hyperviscosity; mut, mutated; PI, proteasome inhibitor; PN, peripheral neuropathy; WM, Waldentstrom’s macroglobulinemia; wt, wild-type.
*Data adapted from Treon, et al.3
For previously treated patients with WM, the genomic-based treatment algorithm (Figure 3) follows a similar process to that of the frontline setting. BTKi monotherapy is the main therapeutic option after first, second, and third relapse in patients with MYD88mut if they have not previously been treated with one; this is due to the high efficacy of BTKis in the R/R setting with both the MYD88mut CXCR4wt genotype.3
If patients with mutated MYD88 or CXCR4 experience disease progression, alternatives such as benda-R and PI-based treatments are used in the first instance if BTKi’s prove ineffective. For later relapses, venetoclax may be considered given its demonstrated durable efficacy in both genotypes. Nucleoside analogs may also be considered for later relapses, although they should not be used in younger patients or those eligible for autologous stem cell transplant, and everolimus, an oral mammalian target of rapamycin inhibitor, is an additional option that can be considered for patients not responding to PI’s, benda-R, or BTKi’s. For patients who experience multiple relapses and chemosensitive disease, autologous stem cell transplantation may be considered as treatment and as a consolidation measure in those with symptomatic amyloidosis. 3
Figure 3. Genomic-based treatment approach for patients with R/R WM*
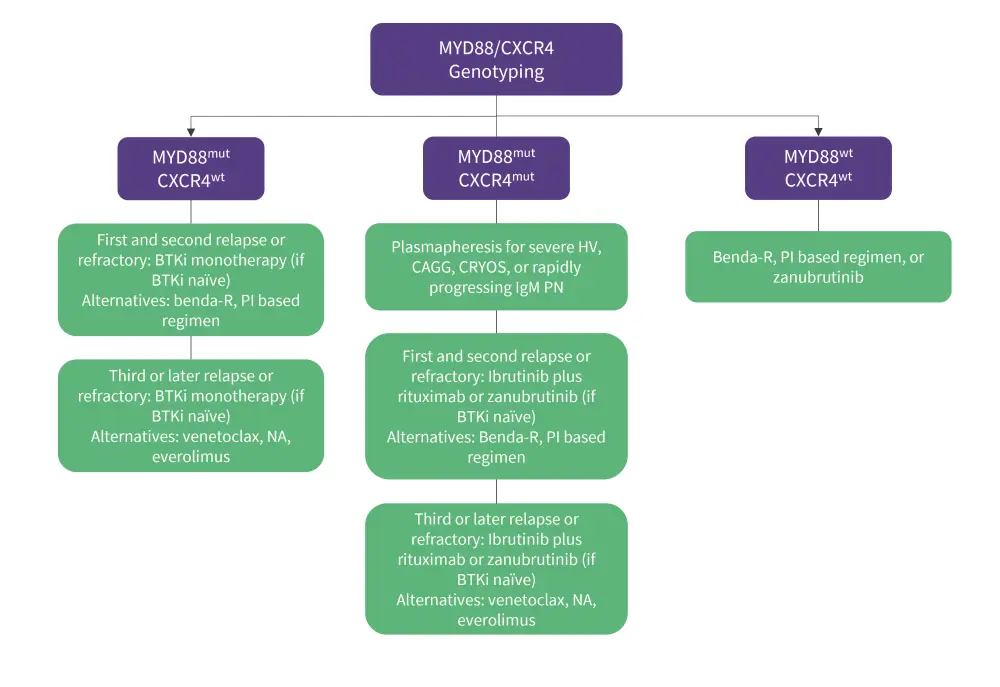
benda R, bendamustine and rituximab; BTKi, Bruton’s tyrosine kinase inhibitor; CAGG, cold agglutinemia; CRYOS, cryoglobulinemia; HV, hyperviscosity; mut, mutated; NA, nucleoside analog; PI, proteasome inhibitor; R/R, relapsed/refractory; WM, Waldenstrom’s macroglobulinemia; wt, wild-type.
*Data adapted from Treon, et al.3
Conclusion
In conclusion, this review reveals the power of genomics as a biomarker and predictor of disease outcomes, as well as a driver in facilitating personalized therapy approaches for patients with WM. BTKis remain a staple treatment regimen given their high efficacy in R/R and frontline settings; however, considering the evolving disease biology and emerging clinical data, novel BTKis currently under development could achieve an optimal effect and address the issues of risk-associated toxicities and resistance.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



