All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Nivolumab with CAR T-cell therapy in progressive DLBCL: final analysis of a phase II study
Chimeric antigen receptor (CAR) T-cell therapy can improve outcomes for patients with diffuse large B-cell lymphoma (DLBCL); however, patients with rapid progressive disease at lymphodepletion have poor outcomes.1 The addition of checkpoint inhibitors targeting PD-1 to CAR T-cell therapy may improve outcomes in patients with DLBCL.1
During the 50th Annual Meeting of the EBMT, Amit presented final results from a phase II trial (NCT05385263) investigating the addition of nivolumab, a checkpoint inhibitor targeting PD-1, to CAR T-cell therapy in patients with DLBCL with progressive disease.1 Below, we summarize the key findings.
Study design1,2
- This study included patients with DLBCL with progressive disease who were enrolled in a CAR T-cell therapy trial.
- Patients received the first dose of nivolumab on Day 5 after CAR T-cell infusion.
- Patients with <100 CAR T cells/μL on Day 7 received a second dose of nivolumab on Day 19.
- Endpoints included safety, response, and durability of response.
Key findings1
Patient characteristics
- In total, 20 patients were included in this trial, of which 12 patients were eligible for nivolumab treatment.
- Eight patients were not eligible to receive nivolumab due to ongoing toxicity following CAR T-cell therapy.
- The median age was 67 years (range, 40–79).
- In total, 95% of patients had progressive disease at lymphodepletion.
Toxicities
In the nivolumab group, 50% of patients experienced post-nivolumab cytokine-release syndrome (Table 1).
Table 1. Toxicity profile*
|
Domain, % |
All patients (n = 20) |
Patients receiving nivolumab (n = 12) |
Patients’ ineligible for nivolumab (n = 8) |
|
CRS, cytokine release syndrome; ICAHT, immune effector cell–associated hematotoxicity; ICANS, immune effector cell associated neurotoxicity syndrome; IEC-HS, immune effector cell–associated hemophagocytic lymphohistiocytosis-like syndrome; IgG, immunoglobulin G; NRM, non-relapse mortality. †All Grade 1–2 CRS. |
|||
|
Overall CRS |
85 |
75 |
100 |
|
Grade 3–4 CRS |
20 |
17 |
25 |
|
Overall ICANS |
20 |
8 |
38 |
|
Grade 3–4 ICANS |
10 |
0 |
25 |
|
Post nivolumab CRS/ICANS |
N/A |
50%† |
N/A |
|
Grade 3–4 ICAHT |
|||
|
Early |
15 |
8 |
25 |
|
Late |
5 |
8 |
0 |
|
Overall IEC-HS |
10 |
8 |
13 |
|
Grade 3–4 IEC-HS |
0 |
0 |
0 |
|
Patients with infections by 3 months |
10 |
17 |
0 |
|
Patients with IgG <3.5 gr/L |
30 |
33 |
25 |
|
NRM at 1 month |
5 |
0 |
13 |
Response
- At 1-month post-CAR T-cell infusion, in all patients, the overall response and complete response rates were 84% and 53%, respectively.
- Response rates were similar between patients who received nivolumab treatment and those who were ineligible due to ongoing toxicities.
Survival outcomes
- There were no major differences in the survival outcomes between patients who received nivolumab vs those who were ineligible for nivolumab.
- The 6- and 12-month survival outcomes for all patients are shown in Figure 1.
Figure 1. 6- and 12-month progression-free survival and overall survival rates*
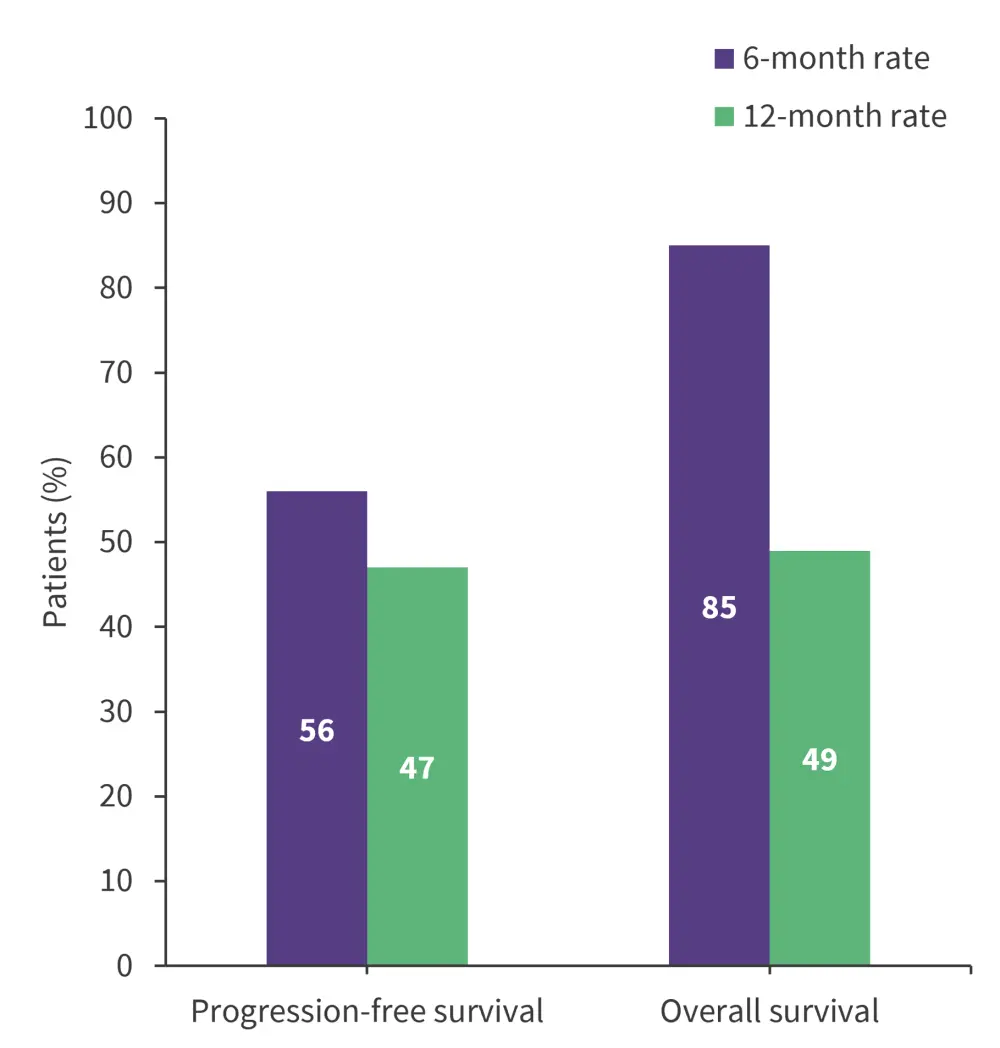
*Data from Amit1
Kinetics
- Patterns of expansion as assessed by quantitative polymerase chain reaction and area under the curve showed no difference between treatment groups.
- There were no differences in CAR-T cell subtypes and the percentage of activation/inhibitory markers in the infused product between treatment groups.
- CAR T-cell subtypes in the peripheral blood at Day 7 were also similar between treatment groups.
- Within the nivolumab group, PD-1 expression was significantly decreased in all mononuclear cells (p = 0.001), in CAR T-cells (p = 0.0034), and in CD3+ non-CAR T-cells (n = 0.001) when compared with patients who did not receive nivolumab.
- CD8+/CD45RO−/CD27+ cells were enriched in both CAR T-cells and CD3+ cells in patients who received nivolumab.
| Key learnings |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content




