All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Novel treatments in R/R CLL: Updates from EHA 2023
Chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) is characterized by repeated relapses which result in poor response to therapy and eventually affect survival. The advent of Bruton’s tyrosine kinase (BTK) inhibitors has transformed the treatment of patients with relapsed/refractory (R/R) CLL/SLL, prolonging progression-free survival (PFS) and overall survival (OS) in patients who otherwise have limited treatment options.
Here, we summarize two key studies on novel treatments for R/R CLL presented at the European Hematology Association (EHA) 2023 Congress; Brown presented results from the phase I/II BRUIN study,1 and Kater presented the final 7-year follow-up and retreatment substudy analysis from the phase III MURANO study.2
Genomic evolution of pirtobrutinib resistance in patients with CLL/SLL pretreated with cBTKi’s: Phase I/II BRUIN study1
The Lymphoma Hub previously reported the efficacy and safety results of phase I/II BRUIN study (NCT03740529) investigating the non-covalent BTKi, pirtobrutinib, for relapsed B-cell malignancies including CLL, SLL, and Waldenstrom’s macroglobulinemia.
Brown presented an analysis that included patients with CLL who were pretreated with covalent BTKis (cBTKis), treated with pirtobrutinib monotherapy in the BRUIN trial, and subsequently experienced disease progression. Targeted next-generation sequencing across all exons of 74 relevant genes, with a limit of detection of 5% variant allele frequency (VAF), was performed on peripheral blood mononuclear cells collected at baseline and at or near progression. Manual inspection of acquired BTK mutations with a limit of detection of 1% VAF was performed at baseline.
Results
Baseline characteristics
Of the 311 patients with CLL enrolled in the BRUIN trial, 49 had progressive disease with available longitudinal paired baseline and progression samples for analysis. The median age was 69 years with a median of four prior lines of systemic therapy; 84% discontinued BTKi due to progressive disease. Among the 49 patients, 90% received prior ibrutinib, 20% received prior acalabrutinib, and 2% received zanubrutinib.
The most common mutations at baseline were BTK (51%), which were mostly C481-related: C481S (n = 23), C481R (n = 4), C481Y (n = 2), C481F (n = 1), and T474l (n = 1). Other common mutations included TP53 (49%), ATM (27%), NOTCH1 (20%), SF3B1 (18%), and PLCG2 (10%). Pirtobrutinib proved effective regardless of the type of prior cBTKi and baseline BTK mutational status, with a best overall response rate (ORR) of 80% versus 82% in the overall cohort.
Genomic characteristics at progression
Among the evaluable patients, 29% had no mutation detected and 71% had at least one acquired mutation at progression, 55% of whom had a BTK mutation. Of the total 82 acquired mutations to pirtobrutinib, the majority were non-C481 BTK mutations, and others included TP53, SF381, PLCG2, BCL2, as well as other mutations, as summarized in Figure 1.
Figure 1. Distribution of acquired mutations at progression*
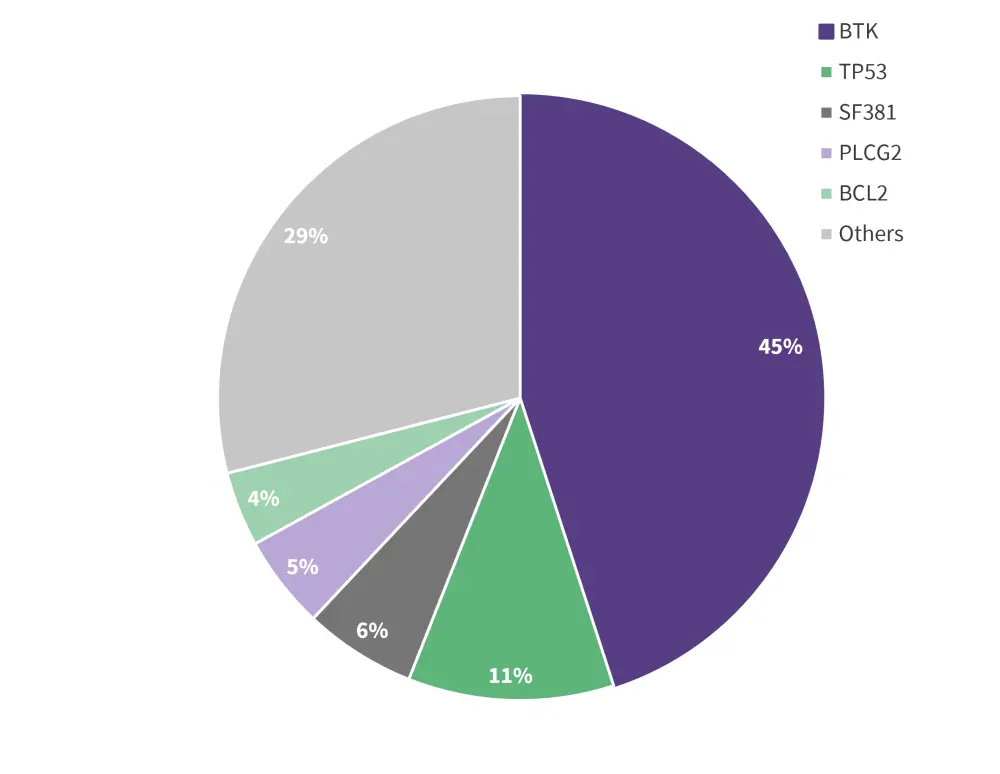
Data from Brown.1
Although there was a decrease in C481 mutations from baseline to progression on pirtobrutinib in 22 /24 patients, three patients acquired C481 mutations (C481R, S, and Y), with the majority of patients acquiring non-C481 mutations such as BTK gatekeeper T474, kinase-impaired L528, and other kinase mutations. The ORR was similar across all subgroups regardless of the acquired BTK mutation (All [86%], BTK C481 [92%], BTK T474 [89%], and BTK other [83%]).
Mutual inspection revealed that 24% of patients had acquired non-C481 BTK mutations at progression that pre-existed at baseline at 1–3% VAF. The best ORR for these patients was 75%, including four who received prior ibrutinib, three receiving acalabrutinib, and one receiving ibrutinib + acalabrutinib.
Presenter’s conclusions
The genomic characteristics of pirtobrutinib resistance included clearance of BTK C481 mutations and emergence of several non-C481 mutations, particularly gatekeeper T474 and kinase-impaired L528W; many acquired mutations pre-existed at the baseline, reflecting emergence on prior cBTKi. Some of the patients did not acquire mutations, suggesting the involvement of alternative resistance mechanisms. Regardless of the type of prior cBTKi and baseline BTK mutational status, pirtobrutinib proved effective across all subgroups of patients with R/R CLL.
7-year follow-up results and substudy analysis of the MURANO trial: Fixed-duration venetoclax + rituximab in R/R CLL2
MURANO (NCT02005471) is a global, open-label, randomized, phase III study evaluating fixed duration venetoclax + rituximab (VenR) vs bendamustine + rituximab (BR) in patients with R/R CLL. We have previously reported the 4- and 5-year results of the MURANO trial on the Lymphoma Hub. Below, we summarize the 7-year efficacy and safety data.
The study endpoints were the updated PFS and OS, time to next treatment (TTNT), and the impact of minimal residual disease (MRD) status on long-term outcomes. The substudy endpoints were ORR, PFS, and MRD evaluation.
Results
At the final data cut-off of August 3, 2022:
- The median PFS and OS benefits with VenR were sustained at 7 years; the 7-year PFS rate was 23% in the VenR group and not estimable in the BR group.
- The estimated 7-year OS was 69.6% vs 51% in the VenR and BR groups, respectively.
- A significantly longer TTNT was observed in the VenR versus BR group, with a median TTNT of 63 months vs 24 months, respectively (p < 0.0001); 95 patients in VenR and 131 in the BR groups received subsequent lines of therapy.
- There were no new safety signals reported from the 5-year follow-up.
- Undetectable MRD (uMRD) was associated with improved PFS following the end of treatment in the VenR arm, with a significantly higher 2-year PFS rate for patients with uMRD compared with high MRD; though this significant association was not found for OS (Table 1).
Table 1. Association of MRD status and PFS outcomes in VenR group*
|
EOT, end of therapy; MRD, minimal residual disease; PFS, progression-free survival; VenR, venetoclax + rituximab. |
||
|
Category† |
Median PFS since EOT |
p value |
|---|---|---|
|
uMRD |
52.5 |
|
|
Low MRD (>10−4 – <10−2) |
29.3 |
p < 0.0001 |
|
High MRD (10−2) |
4.6 |
p < 0.0001 |
Of the 83 patients with uMRD at the end of therapy, one patient died, five had progressive disease, 14 had sustained uMRD, and 63 had MRD conversion. The proportion of patients transitioning after end of therapy to MRD conversion to progressive disease or death, and ultimately second line treatment are summarized in Figure 2; there was a 4-year period for this process. The 14 patients with sustained uMRD at the end of therapy had favorable baseline characteristics, including wild-type TP53 and mutated IGHV.
Figure 2. Proportion of patients in MRD conversion who progressed and received second-line treatment*
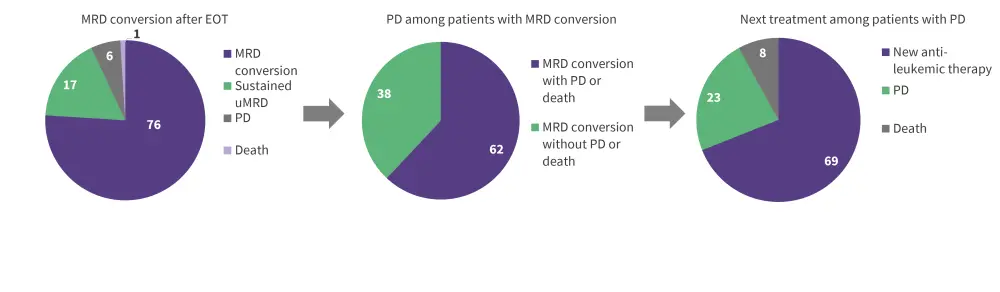
EOT, end of therapy; MRD, minimal residual disease; PFS, progression-free survival; VenR, venetoclax + rituximab.
*Data from Kater.2
†For patients who completed 2 years of VenR without disease progression.
Substudy results
Of the 34 patients included in the substudy analysis, 25 were retreated with VenR and categorized as high-risk. Baseline characteristics for these patients are summarized in Table 2.
Table 2. Baseline patient characteristics*
|
CIRS, cumulative illness rating score; IGHV, immunoglobulin heavy chain variable region; TLS, tumor lysis syndrome |
|
|
Characteristic, % (unless otherwise stated) |
Venetoclax-obinutuzumab |
|---|---|
|
Median age, years |
66 (49−82) |
|
Number of prior lines of therapy† |
|
|
2 |
80 |
|
3 |
16 |
|
≥4 |
4 |
|
Del (17p)‡ |
|
|
Deleted |
28 |
|
Not deleted |
32 |
|
Unknown/not assessed |
40 |
|
TP53 status§ |
|
|
Mutated |
20 |
|
Unmutated |
68 |
|
Unknown/not assessed |
12 |
|
IGHV status‖ |
|
|
Mutated |
4 |
|
Unmutated |
88 |
|
Unknown/not assessed |
8 |
|
Genomic complexity |
|
|
0–2 |
36 |
|
3-4 |
12 |
|
≥5 |
32 |
|
Unknown/not assessed |
20 |
At the median follow-up of 33.4 months,
- median PFS was 23.3 months;
- best ORR was 72%, with 24% CR rate;
- median OS was not reached; and
- 8% of patients achieved uMRD at the end of combination therapy; however, this was not sustained for the duration of treatment, with no patients retaining their uMRD status at the end of retreatment.
Conclusion
The final 7-year follow-up analysis of the MURANO trial demonstrated sustained PFS and OS benefits and a longer TTNT for VenR compared to BR in patients with R/R CLL. This trial also showed the predictive value of uMRD, which was associated with prolonged PFS in the VenR arm. In the substudy analysis, retreatment with VenR yielded high response rates and uMRD was attainable in this high-risk population, thus Ven R is a viable option in R/R CLL.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content




