All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Optimal management of patients with R/R PTCL
Do you know... Which of the following treatments was removed from fast-track designation by the U.S. FDA in 2021 but is still recommended under NCCN guidelines?
Peripheral T-cell lymphomas (PTCL) are a group of rare heterogeneous non-Hodgkin lymphomas originating from mature T cells.1,2 Currently, survival outcomes are poor for most patients with PTCL and the 5-year overall survival rate is ~30–35%.1 Currently, there is no current standard-of-care approach for treating relapsed/refractory (R/R) PTCL; however, several novel treatments are being investigated to improve survival outcomes.1,2
The Lymphoma Hub previously published an expert opinion on the treatment horizon for PTCL. Here, we summarize two studies; one by Braunstein et al.,1 published in Journal of Personalized Medicine; and the other by Stuver and Moskowitz,2 published in Cancers; discussing the advances in the management of patients with R/R PTCL.
Currently approved treatments for R/R PTCL
Currently, there are two primary categories of R/R PTCL treatment, a single-agent treatment or salvage therapy to prepare for allogeneic hematopoietic stem cell transplant (allo-HSCT); however, progression-free survival is poor (Figure 1).
Figure 1. Approved therapies for R/R PTCL*
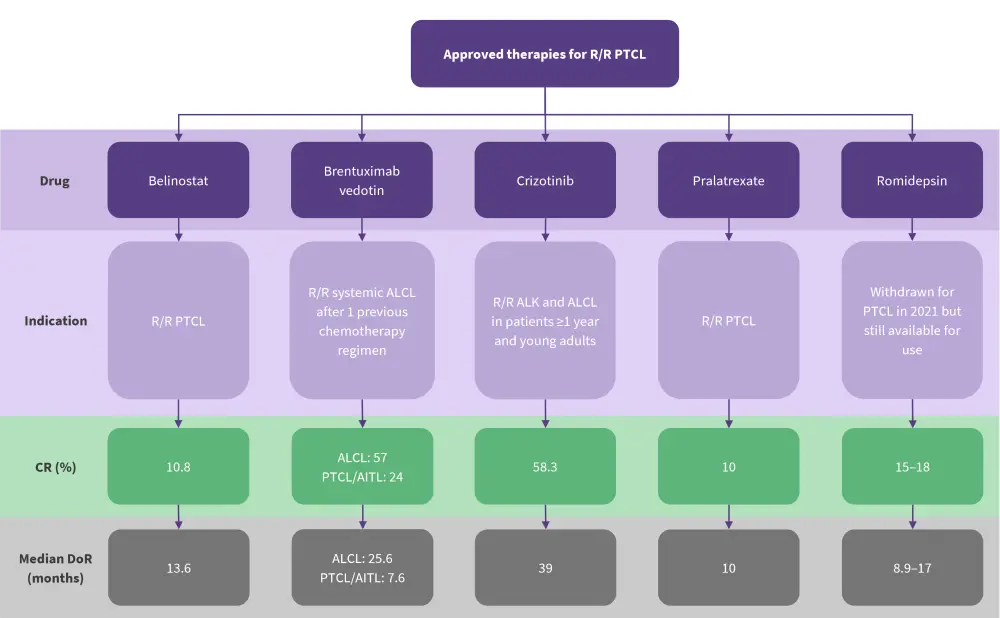
ALK, anaplastic lymphoma kinase; AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large cell lymphoma; CR, complete response; DoR, duration of response; PTCL, peripheral T-cell lymphoma; R/R, relapsed/refractory.
*Adapted from Stuver and Moskowitz.2
Treatment selection depends on various factors, such as age, performance status, disease status, comorbidities, and organ function. In previous chemotherapy-intensive regimens, the influence of age on treatment selection would depend on toxicity profiles; however, novel treatments should not be limited by age.1 For example, romidepsin and belinostat are standard-of-care treatments for patients with angioimmunoblastic T-cell lymphoma (AITL), and bendamustine is preferred as a bridging therapy to allo-HSCT in patients who are older, or in those with a poor performance status who require low toxicity.
Romidepsin, although withdrawn from accelerated approval by the U.S. Food and Drug Administration in 2021, is still recommended for use under the National Comprehensive Cancer Network guidelines; it remains commercially available in the United States. It is now also being investigated as combination therapy.
Monotherapy
There are several studies investigating monotherapy/single-agent treatments for patients with R/R PTCL (Table 1).
Table 1. Investigational monotherapies for R/R PTCL*
|
CR, complete response; DoR, duration of response; NR, not reported; PTCL, peripheral T-cell lymphomas; R/R, relapsed/refractory. |
||||
|
Drug |
Trial number |
Trial stage |
CR, % |
Median DoR, |
|---|---|---|---|---|
|
AFM13 |
II |
NR |
NR |
|
|
CTX130 |
I |
30 |
NR |
|
|
Duvelisib |
II |
34 |
7.7 |
|
|
Golidocitinib |
I/II |
22 |
NR |
|
|
Pembrolizumab |
II |
NR |
NR |
|
|
TT1621 |
I |
3 |
5.9† |
|
|
Tenalisib |
I |
9 |
4.9 |
|
|
Valemetostat |
II |
24 |
12.9 |
|
Targeting oncogenic pathways involved in T-cell receptor and cytokine signaling has been observed to be an effective therapeutic strategy.2 Duvelisib targets the phosphoinositide 3-kinase (PI3K)/protein kinase B/mammalian target of rapamycin pathway, playing a critical role in T-cell receptor signaling, and has been listed by the National Comprehensive Cancer Center as a treatment option for R/R PTCL.1,2 When brentuximab vedotin fails to yield a response in patients with anaplastic large cell lymphoma, duvelisib can be used, as demonstrated by promising results from the PRIMO trial (NCT03372057). Further analysis from this trial is yet to be carried out to fully elucidate subtype specific responses, as well as the ability to bridge to allo-HSCT.1,2
Based on encouraging outcomes from a phase I/II clinical trial (NCT04105010), golidocitinib has been granted fast-track designation by the U.S. Food and Drug Administration.2 Tenalisib, a PI3K inhibitor, has also shown potential as a monotherapy and is being investigated as a combination treatment with romidepsin to manage toxicity.2
Novel combination therapies
To date, no combination therapy has shown superiority and none are approved for use. However, the therapeutic field is expanding, and several combination treatments are being investigated (Table 2).
Table 2. Investigational combination treatments for R/R PTCL*
|
CR, complete response; DoR, duration of response; NR, not reported; PTCL, peripheral T-cell lymphomas; R/R, relapsed/refractory. |
|||
|
Drug |
Trial stage |
CR, % |
DoR, months |
|---|---|---|---|
|
Nanatinostat + valganciclovir |
I/II |
19 |
10.4 |
|
Romidepsin + duvelisib |
I |
44 |
NR |
|
Romidepsin + tenalisib |
I/II |
26 |
5 |
Romidepsin, in combination with the PI3K inhibitor duvelisib, has shown encouraging survival rates in patients with R/R PTCL2; this treatment has also enabled bridging to allo-HSCT. Similarly, romidepsin in combination with tenalisib has shown high response rates.
Nanatinostat + valganciclovir has shown efficacy as a combination treatment in patients with Epstein-Barr virus-positive lymphomas by inhibiting class I selective histone deacetylase and acting as an antiviral.1,2 Patients with AITL also responded well to this treatment (Table 2).2
Allo-HSCT and auto-HSCT
Although patients with PTCL are benefiting from advances in the therapeutic landscape, the survival rates are not as durable as allo-HSCT. There is uncertainty around stopping treatment in patients who have achieved a disease-free status. In a recent study by the European Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research, the 3-year progression-free survival was 48–52% in patients undergoing allo-HSCT. Currently, no other treatment showed similar survival in patients with PTCL; particularly, PTCL not otherwise specified, AITL, and anaplastic large cell lymphoma.
The role of autologous-HSCT remains unclear, although it has shown improved survival with lower non-relapse mortality compared with allo-HSCT in patients with chemo-sensitive disease (86% vs 60%, respectively). This demonstrates that the specific type of PTCL impacts survival response and therefore, should continue to be a factor in treatment decisions.1,2
Conclusion
Although its heterogeneity and rarity make treatment of R/R PTCL challenging, the therapeutic field is continuously expanding.1,2 Novel agents, such as duvelisib and ruxolitinib, targeting PI3K/protein kinase B/mammalian target of rapamycin and Janus kinase/signal transducers and activators of transcription pathways, are showing promising outcomes. Alongside these single-agent novel treatment, strategies to bridge patients to allo-HSCT are also underway as a potential curative treatment option for patients who are transplant eligible.1,2
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


