All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Results from phase I ALEXANDER study investigating the first CAR T-cell therapy, AUTO3, plus pembrolizumab to target both CD19 and CD22 in the treatment of R/R DLBCL
Available CD19 chimeric antigen receptor (CAR) T-cells show activity in relapsed lymphoma, but long-lasting complete response rates remain below 40%. PD-L1 upregulation, that results in T-cell exhaustion, and CD19 antigen loss are thought to play a role in relapse.1 In the phase I/II ALEXANDER trial (NCT03287817), Aravind Ramakrishnan and colleagues investigated the potential for targeting both CD19 and CD22 simultaneously to reduce antigen escape, and the effect of pre-conditioning with pembrolizumab on T-cell exhaustion in the treatment of relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL). The results from phase I were presented by Ramakrishnan during the American Society of Clinical Oncology (ASCO) 2020 Virtual Annual Meeting, and the data are summarized here.1
AUTO3 is innovative with its bicistronic structure, and one gamma-retroviral encoding two CARs optimized to target CD19 and CD22 independently. Humanized binders were designed to cause less toxicity and the OX40 (for CD19) and 41BB (for CD22) costimulatory domains to improve in vivo persistence.1
Study design1
- Single arm, open-label, multi-center, phase I/II study (see Figure 1)
Figure 1. Summary of study design1
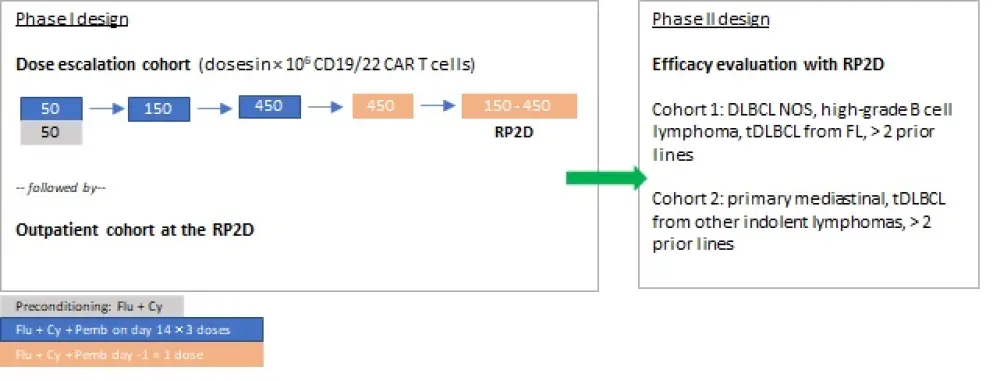
Cy, cyclophosphamide; DLBCL, diffuse large B cell lymphoma; FL, follicular lymphoma; Flu, fludarabine; NOS, not otherwise specified; Pemb, pembrolizumab; RP2D, recommended phase 2 dose; tDLBCL, transformed DLBCL
Patients were deemed eligible if they met the following criteria:
- Aged ≥ 18 years
- Refractory disease after chemotherapy, or relapsed following ≥ 2 lines of therapy, or autologous stem cell transplantation (auto-SCT)
- DLBCL NOS, DLBCL with MYC and BCL2 and/or BCL6 rearrangements
- tDLBCL from FL or other indolent lymphomas (except Richter’s transformation)
- High-grade B cell lymphoma with MYC expression (except Burkitt’s lymphoma)
- Primary mediastinal large B cell lymphoma
Patients with previous treatment with allogeneic hematopoietic stem cell transplant, and CD19- or CD22-targeted therapy were excluded.
Primary endpoints:
- Phase I: Incidence of Grade 3–5 toxicity within 75 days of AUTO3 infusion and dose limiting toxicities
- Phase II: Overall response rate following AUTO3 infusion and incidence of Grade 3–5 toxicity within 75 days post-infusion
Secondary endpoints for phase I and II:
- Safety, feasibility of AUTO3 product generation, cellular kinetics, complete response, disease-free survival, progression-free survival, overall survival, and B-cell aplasia
Patient characteristics1
Among 23 evaluable patients, median age was 57 years (range, 28–83) and the majority of patients were male (n = 14). The median number of prior therapies was three (range, 2–10), and four patients had previously received auto-SCT. The majority of patients (16/23) had Stage IV disease, indicating a very high-risk patient population. Other patient characteristics are summarized in Table 1, below.
Table 1. Baseline characteristics1
|
DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; GCB, germinal center B cell-like; IPI, International Prognostic Index; MZL, marginal zone lymphoma; SPD, sum of the product of the diameters; tDLBCL, transformed DLBCL; R/R, relapsed/refractory |
|
|
Characteristic |
N = 23 |
|---|---|
|
Histology, n DLBCL
tDLBCL
|
10 7
5 1 |
|
R/R disease, n Refractory Relapsed R/R |
5 3 15 |
|
IPI, n 0–1 2 3–4 |
4 7 12 |
|
Median SPD, cm (range) |
22.3 (2.08–260.84) |
Safety1
Among evaluable patients (n = 23), most Grade ≥ 3 treatment-emergent adverse events (≥ 25% occurrence) were hematological events, and there were no dose limiting toxicities, deaths or Grade 5 adverse events related to AUTO3 infusion. Grade 3–4 neutropenia, thrombocytopenia, and anemia occurred in 87%, 57%, and 48% of patients, respectively. Serious adverse events were mostly hematologic events, and others included gallbladder abscess (n = 1), Grade 4 parainfluenza pneumonia (n = 1), subdural hemorrhage (n = 1), and Grade 3 neurotoxicity (n = 1); all of which resolved.
Cytokine release syndrome
Cytokine release syndrome (CRS) events occurred in nine patients (39%). Grade 1 and 2 CRS occurred in six and three patients, respectively. No Grade ≥ 3 CRS occurred with primary infusion. Tocilizumab was administered to four patients. Median time to onset of CRS was 7 days (range, 1–36) with a median duration of 5 days (range, 1–19).
Neurotoxicity
One patient experienced neurotoxicity on Day 53, which was Grade 3, and occurred in the 50 × 106 AUTO3 without pembrolizumab cohort. The patient was managed with steroid treatment and the event resolved. Of note, no neurotoxicity events were seen in the 150 × 106 AUTO3 and 450 × 106 AUTO3 cohorts, as well as pembrolizumab cohorts.
Efficacy1
Overall response rate (ORR) and CR rates among evaluable patients are presented in Table 2, below.
Table 2. Response rates
|
CR, complete response; ORR, overall response rate; pem, pembrolizumab |
|||
|
Outcome |
All dose levels (n = 23) |
Doses of ≥ 150 × 106 (n = 16) |
≥ 150 × 106 plus Day −1 pem (n = 8) |
|---|---|---|---|
|
ORR, % |
65 |
69 |
75 |
|
CR, % |
48 |
56 |
63 |
Complete remission was achieved in 11/23 patients. At a median follow-up of 3 months (range, 1–12), complete responses achieved with ≥ 150 × 106 AUTO3 doses were durable, which were considered a possible effect of dual targeting.
The recommended phase II dosing range of 150–450 × 106 dose with pembrolizumab on Day −1 was selected.
Conclusion
These data indicate durable complete responses with a favorable safety profile, with a low incidence of Grade 3 and higher CRS and neurotoxicity events. According to the investigators, one possible reason behind low levels of CRS and neurotoxicity events is the lower levels of cytokine production due to humanized binders, novel spacers, and the costimulatory domains in AUTO3, but this needs further evaluation. Now that the dose escalation phase has completed and a dose between 150 × 106 and 450 × 106 AUTO3 plus Day −1 pembrolizumab has been recommended for phase II, the outpatient expansions cohort will begin enrollment soon.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

