All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Roche, Sobi and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Incyte, and Lilly. The funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Do you know... In the phase II CAPTIVATE trial, treatment with ibrutinib + venetoclax achieved the numerically highest PFS rate in patients with which cytogenetic feature?
Symposium | First-line BTK inhibitors for CLL: Current and future perspectives
During the Lymphoma Hub virtual symposium on October 23, 2024, entitled Customizing first-line Bruton‘s tyrosine kinase (BTK) inhibitors for chronic lymphocytic leukemia (CLL), Paolo Ghia, Università Vita-Salute San Raffaele, RCCS Ospedale San Raffaele, Milano, IT, delivered a presentation on the current and future perspectives of BTK inhibitors in first-line (1L) CLL.
Ghia provided an overview of three generations of BTK inhibitors for the 1L treatment of CLL in the US and Europe, including their regulatory status. He emphasized the advantages of fixed-duration therapy, such as minimal side effects, a reduced risk of clonal evolution and resistance, lower financial costs, the possibility of retreatment, and improved quality of life. Additionally, he highlighted the importance of combining venetoclax, a B-cell lymphoma (BCL)-2 inhibitor, with BTK inhibitors and anti-CD20 monoclonal antibodies to achieve deep and lasting responses.
Ghia shared the latest data from clinical trials of fixed-duration BTK inhibitors as doublet and triplet combination therapies in 1L CLL, and presented key outcomes from the CAPTIVATE, GLOW, CLL13/GAIA, NCT03580928, and AMPLIFY trials (Figure 1). He then discussed the importance of measurable residual disease (MRD)-guided fixed-duration approaches in the 1L CLL treatment paradigm (Figure 2), using clinical data from the BoVen and FLAIR trials, and talked about how MRD-guided treatment allows tailored therapy duration based on the MRD status.
Finally, Ghia considered future trials of emerging doublet and triplet combinations of BTK inhibitors, including the CLL16, CLL17, MAJIC, and RESOLVE trials, with new oral therapies and next-generation anti-CD20 antibodies as promising options for patients with high-risk disease features.
Figure 1. Key outcomes from fixed-duration doublet and triplet combination trials in 1L CLL*

1L, first line; 2L, second line; CI, confidence interval; CIT, chemoimmunotherapy; CK, complex karyotype; Clb, chlorambucil; CLL, chronic lymphocytic leukemia; CR, complete response; CRi, CR with incomplete bone marrow recovery; HR, hazard ratio; Ibr, ibrutinib; IGHV, immunoglobulin heavy chain variable region; mIGHV, mutated IGHV; MRD, measurable residual disease; Ob, obinutuzumab; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PR, partial response; R, rituximab; uIGHV, unmutated IGHV, uMRD, undetectable MRD; Ven, venetoclax.
*Data from Wierda1, Moreno et al.2, Eichhorst et al.3, and Davids et al.4
Figure 2. Significance of MRD-guided approaches in fixed-duration therapy*
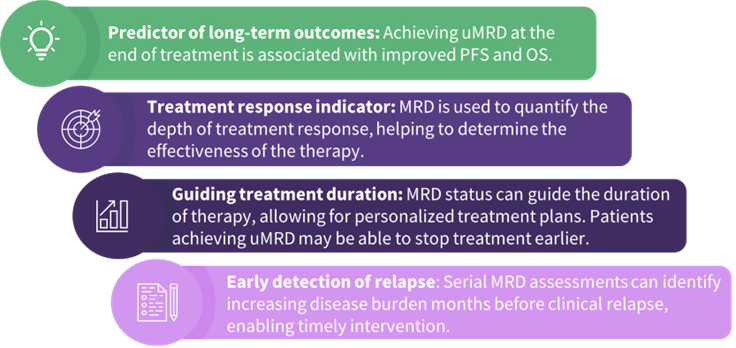
MRD, measurable residual disease; OS, overall survival; PFS, progression-free survival; uMRD, undetectable MRD.
*Adapted from Seymour, et al.5
| Key learnings |
|
The use of new oral, targeted therapies and next-generation anti-CD20 monoclonal antibodies has significantly improved outcomes for treatment-naïve patients with CLL. |
|
Doublet and triplet combination regimens have shown encouraging outcomes, leading to fixed-duration regimens becoming potential alternatives to continuous therapy.
|
|
MRD-guided treatment represents a promising approach for managing CLL, offering the capability for more effective and less toxic therapy. |
This independent educational activity was supported by an educational grant from Janssen Biotech, Inc., administered by Janssen Scientific Affairs, LLC. All content was developed independently by SES in collaboration with the faculty. The funder was allowed no influence on the content of this activity.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
In your experience, when do most CRS/ICANS events occur after lisocabtagene maraleucel infusion?


 Paolo Ghia
Paolo Ghia


