All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Anti-CD19 CAR T-cell therapy for patients with primary or secondary CNS lymphoma
Patients with relapsed/refractory (R/R) primary central nervous system lymphoma (PCNSL) or secondary central nervous system lymphoma (SCNSL) have a dismal prognosis; as such, there is a need for novel treatment strategies for these patients.1 Several trials, including ZUMA-1 (NCT02348216) and JULIET (NCT02445248), have demonstrated the efficacy of anti-CD19 chimeric antigen receptor (CAR) T-cell therapy in patients with relapsed/refractory large B-cell lymphoma1; however, there is a lack of data on the safety and efficacy of anti-CD19 CAR T-cell therapy in patients with PCNSL/SCNSL.1
During the 50th Annual Meeting of the European Society for Blood and Marrow Transplantation (EBMT), Ossami Saidy presented results from an analysis of CD19-targeted CAR T-cell therapy in patients with PCNSL or SCNSL from the EBMT and GoCART coalition.1 We summarize this presentation below.
Study design1
- This analysis included adult patients aged ≥18 years with PCNSL or SCNSL who received their first CAR T-cell therapy between January 2018 and February 2023.
- The primary endpoints were overall survival and progression-free survival.
- Secondary endpoints included relapse incidence, non-relapse morality, and toxicity.
- Data was obtained from the EBMT registry and a questionnaire.
Key findings1
Patient characteristics
A total of 95 patients with a median age of 62.6 years were included in this analysis. (Table 1).
Table 1. Patient characteristics*
|
Auto-HSCT, autologous hematopoietic stem cell transplantation; CAR, chimeric antigen receptor; CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; PCNSL, primary central nervous system lymphoma; SCNSL, secondary CNSL. *Adapted from Ossami Saidy.1 |
|
|
Patient characteristics, % (unless otherwise specified) |
All patients (N = 95) |
|
Median age, years (range) |
62.6 (23–80) |
|
Aged >70 years |
25 |
|
Gender |
|
|
Male, n |
55 |
|
Female, n |
40 |
|
Type of CNS lymphoma |
|
|
PCNSL |
10.5 |
|
SCNSL |
89.5 |
|
ECOG Performance Status ≥2† |
22.6 |
|
Number of prior lines of therapy‡ |
|
|
1 |
5.8 |
|
2 |
30.4 |
|
≥3 |
63.8 |
|
Previously auto-HSCT§ |
32.3 |
|
Remission status at CAR T-cell infusion‖ |
|
|
Stable disease/progressive disease |
67.4 |
|
Partial remission |
24.7 |
|
Complete remission |
7.9 |
Survival outcomes
- After a median follow-up of 20.1 months, the 2-year progression-free survival and overall survival rates were 38.1% and 48.4%, respectively (Figure 1).
- Patients who received CAR T-cell therapy in complete or partial remission had improved survival outcomes compared with patients who had stable or progressive disease (Figure 1).
Figure 1. 2-year survival rates in patients with PCNSL or SCNSL*
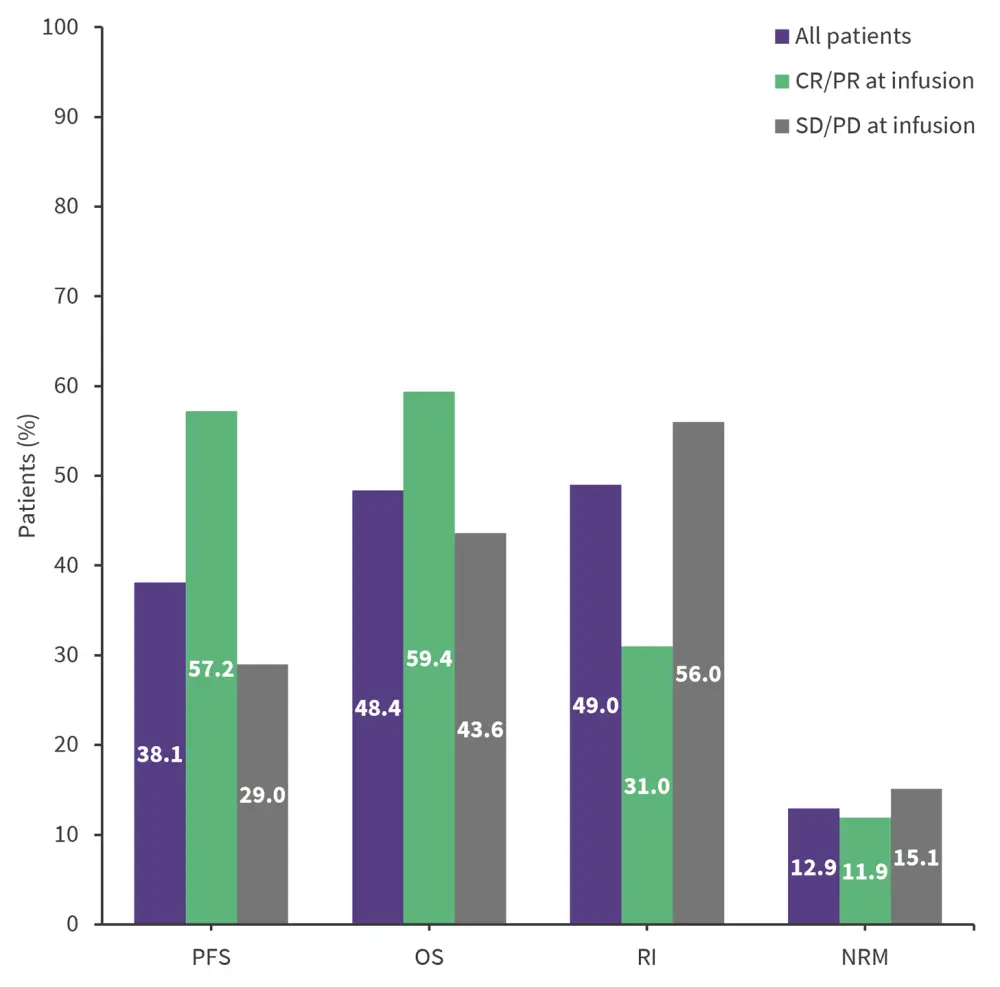
CR, complete remission; NRM, non-relapse mortality; OS, overall survival; PCNSL, primary central nervous system lymphoma; PD, progressive disease; PFS, progression-free survival; PR, partial remission; RI, relapse incidence; SCNSL, secondary central nervous system lymphoma; SD, stable disease.
*Data from Ossami Saidy1
Toxicities
After a median follow-up of 27 months, the incidence rates of immune effector cell-associated neurotoxicity syndrome at 3 and 15 days were 17% and 43%, respectively.
| Key learnings |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content





