All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Anti-CD19 monoclonal antibodies for the treatment of DLBCL
Featured:
Do you know... In the phase II L-MIND trial, what was the 5-year overall response rate for patients with R/R DLBCL treated with the tafasitamab plus lenalidomide regimen?
During the Lymphoma Hub Steering Committee meeting, Grzegorz Nowakowski, the Mayo Clinic, Rochester, US, chaired a discussion on anti-CD19 monoclonal antibodies for the treatment of diffuse large B-cell lymphoma (DLBCL). This discussion also featured Catherine Thieblemont, Francesc Bosch, Gilles Salles, Marek Trněný, Michael Dickinson, Miles Prince, Sonali Smith, and Ulrich Jäger.
Anti-CD19 monoclonal antibodies for the treatment of DLBCL
Anti-CD19 monoclonal antibodies for the treatment of DLBCL
Nowakowski started by presenting an overview of tafasitamab (Tafa), a CD19 monoclonal antibody, as a potential therapeutic agent for B-cell malignancies, including the complementary mechanisms of action of Tafa and lenalidomide (Len) (Figure 1). Tafa-Len demonstrated significant clinical benefit in the phase II L-MIND trial (NCT02399085), achieving a high overall response rate (ORR) and durable remissions in patients with relapsed/refractory (R/R) DLBCL, particularly in the second-line setting (Figure 2). Real-world evidence (RWE) broadly confirmed these findings, especially among patients who met the L-MIND eligibility criteria (Table 1). Nowakowski also shared promising early data on detectable CD19 expression following Tafa in patients with R/R DLBCL.
Figure 1. Tafa-Len: Mechanism of action*
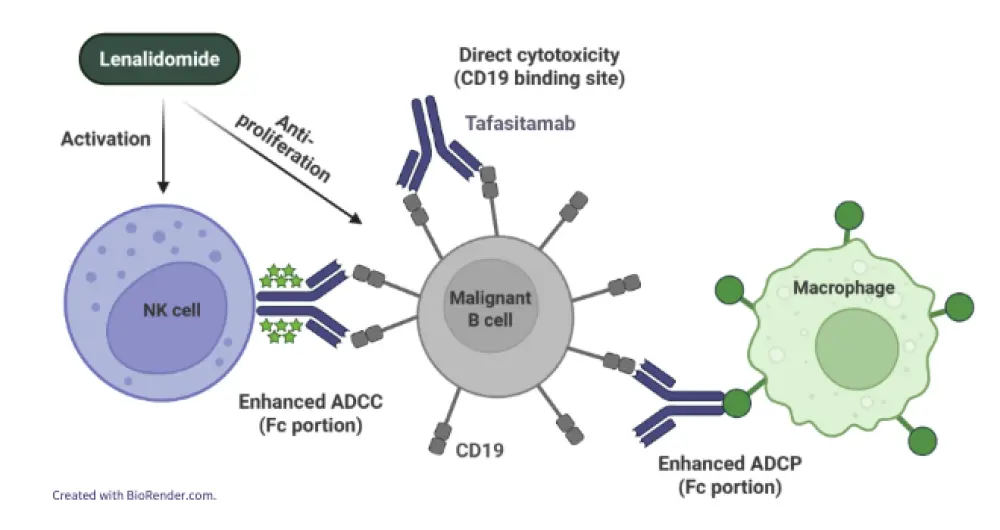
Figure 2. Efficacy of Tafa-Len in R/R DLBCL: 5-year data from the L-MIND trial*
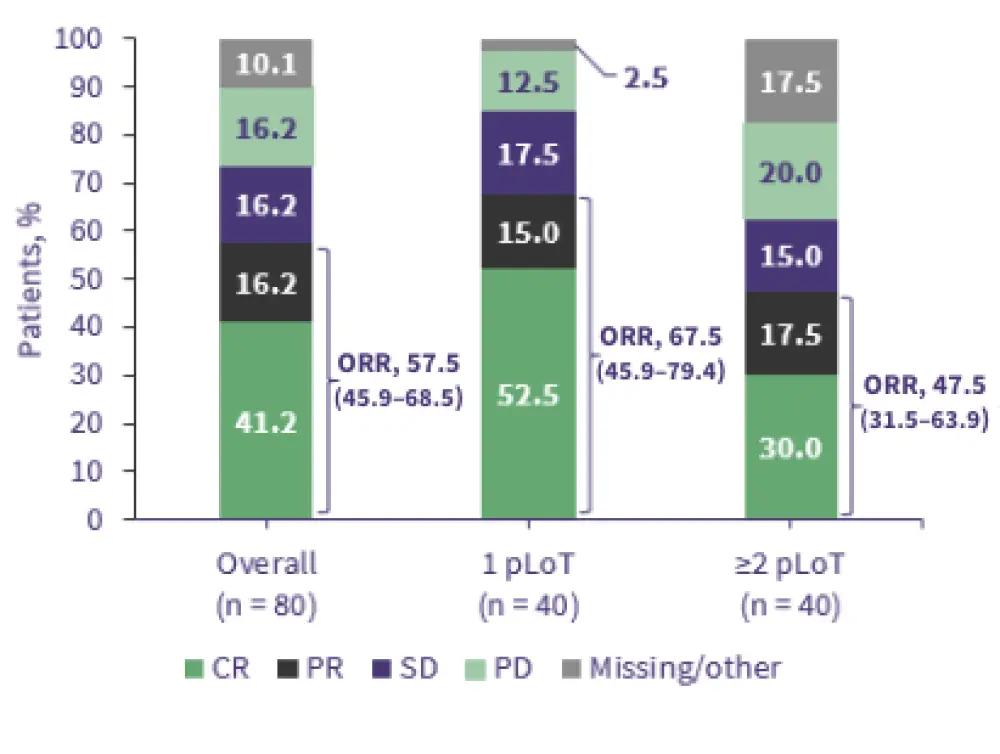
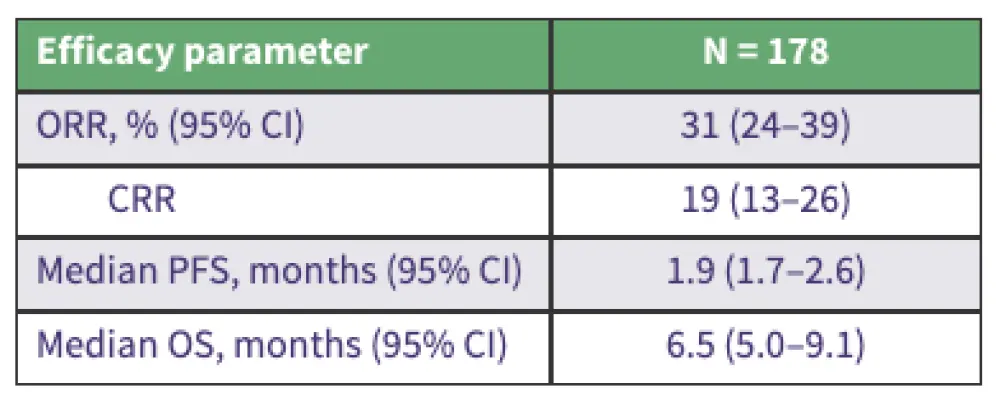
Table 1. RWE of Tafa-Len in R/R DLBCL*
Looking forward, Nowakowski introduced ongoing studies, including the FrontMIND (NCT04824092), ROBUST (NCT02285062), ECOG-ACRIN E1412 (NCT01856192), LOTIS-2 (NCT03589469), LOTIS-5 (NCT04384484), and LOTIS-7 (NCT04970901) trials investigating anti-CD19 monoclonal antibodies and anti-CD19 drug conjugates in combination with other anticancer agents in the R/R non-Hodgkin lymphomas. This presentation was followed by a panel discussion.
Key learnings
CD19 as a therapeutic target
CD19 has emerged as a clinically important target. It is now central to several therapeutic classes, including monoclonal antibodies (e.g., Tafa), antibody–drug conjugates (e.g., loncastuximab), and bispecific/trispecific antibodies.
L-MIND trial and regulatory impact
The pivotal phase II L-MIND study evaluated Tafa-Len in R/R DLBCL:
- With a median follow-up of 13.2 months, the ORR was 60%.
- These results led to the accelerated approval of the Tafa-Len regimen for the treatment of patients with R/R DLBCL ineligible for high-dose chemotherapy or transplant.
- At 5 years, ORR was 57.5%, median progression-free survival (PFS) was 11.6 months, and median overall survival (OS) was 33.5 months.
Real-world evidence
Real-world outcomes of Tafa-Len in patients with R/R DLBCL:
- The ORR was 31%, median PFS was 1.9 months, and median OS was 6.5 months.
- PFS and OS rates were significantly higher in patients who met the L-MIND eligibility criteria.
Real-world outcomes of Tafa in patients with R/R DLBCL in the US:
- The ORR was 73.5% (overall), 78.5% (Tafa as second line), 62.8% (Tafa as third line).
- The PFS was 11.3 months, and OS was 24.8 months.
- Uptake was increased in second-line settings, especially where CAR T-cell therapies or bispecific antibodies are not available.
CD19 expression is preserved post-Tafa exposure
Data suggest that CD19 expression remains detectable even after Tafa exposure, supporting subsequent use of CD19-targeting CAR T-cell therapy. Ongoing trials are exploring Tafa as a bridge to CAR T-cell therapy, possibly improving CAR-T expansion by modulating early T-cell activation.
Clinical uptake and positioning
Tafa-Len is gaining momentum as a second-line option for patients with R/R DLBCL, particularly in patients who are ineligible for CAR T-cell therapy or bispecific antibodies. Its value is especially recognized in community practice, though evolving access to novel agents may influence future use.
Importance of combination therapy
Tafa monotherapy is suboptimal, and combination with Len is essential to achieve meaningful efficacy. Importantly, logistical delays in Len access should not delay therapy initiation with Tafa-Len.
Lenalidomide dosing in practice
The approved 25 mg dose of Len is frequently reduced in real-world settings due to toxicities such as cytopenia, renal impairment, or frailty. Many clinicians initiate at 10–20 mg. Even lower doses may provide a durable benefit in select patients. There is consensus on the need for dosing optimization studies.
Patient selection
Tafa-Len is especially suitable for patients relapsing >12 months after first-line therapy who face access barriers to CAR T-cell therapy. However, such patients are becoming less common in current practice, and trial populations may not reflect real-world settings.
Treatment duration and administration schedule
There is growing concern about the burden of indefinite therapy and weekly administrations in early cycles of therapy. Fixed-duration treatment strategies and revised schedules can improve patient convenience. Current pharmacokinetic/pharmacodynamic data guiding these schedules (weekly administration during Cycles 1–3 followed by administration every two cycles) are limited.
Optimizing Tafa-Len in first-line DLBCL
Building on earlier Len studies (the phase III ROBUST [NCT02285062] and phase II ECOG-ACRIN E1412 [NCT01856192] trials), the First-MIND trial compared R-CHOP + Tafa vs Tafa-Len. The R-CHOP + Tafa doublet showed improved early efficacy without dose delays. This informed the ongoing phase III Front-MIND trial (R-CHOP ± Tafa-Len) in high-risk DLBCL (IPI ≥3, rapid progression).
In summary, Tafa-Len remains a meaningful treatment for R/R DLBCL, particularly in second-line settings and for patients who are ineligible for CAR T-cell therapies. Its future positioning will depend on first-line data (e.g., from FrontMIND), and addressing practical challenges around duration, scheduling, and sequencing with other therapies.
This educational resource is independently supported by Incyte. All content was developed by SES in collaboration with an expert steering committee; funders were allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Ulrich Jäger
Ulrich Jäger Grzegorz Nowakowski
Grzegorz Nowakowski Michael Dickinson
Michael Dickinson Marek Trněný
Marek Trněný Catherine Thieblemont
Catherine Thieblemont Gilles Salles
Gilles Salles Francesc Bosch
Francesc Bosch Sonali Smith
Sonali Smith Miles Prince
Miles Prince


.webp&w=3840&q=75)