All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Novel and emerging treatment options for R/R DLBCL
Do you know... Which of the following was the first CAR T-cell therapy approved by the FDA for the treatment of patients with R/R DLBCL?
Diffuse large B-cell lymphoma (DLBCL) is one of the most common subtypes of aggressive lymphoma.1,2 Depending on individual risk factors, around 60–65% of patients can be treated by chemoimmunotherapy with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), while the remaining patients experience primary refractory/relapsed (R/R) disease.1,2 Patients with transplant-eligible R/R DLBCL have typically been treated with intensive salvage regimens followed by high-dose chemotherapy (HDT) and autologous stem cell transplantation (ASCT); however, this only cures a limited number of patients.1 Furthermore, those who are ineligible for ASCT have a poor prognosis, representing an important unmet need for patients with R/R DLBCL.2 In recent years, a plethora of novel agents including monoclonal antibodies (mAbs), antibody–drug conjugates (ADCs), immune checkpoint inhibitors, monospecific and bispecific mAbs, and chimeric antigen receptor (CAR) T-cell therapies have emerged as potential alternatives to conventional therapies for patients with R/R DLBCL. Here, we provide an overview of the novel and emerging treatment options for R/R DLBCL, as illustrated in Figure 1.
Figure 1. Recently approved novel agents and emerging promising treatment options for R/R DLBCL*
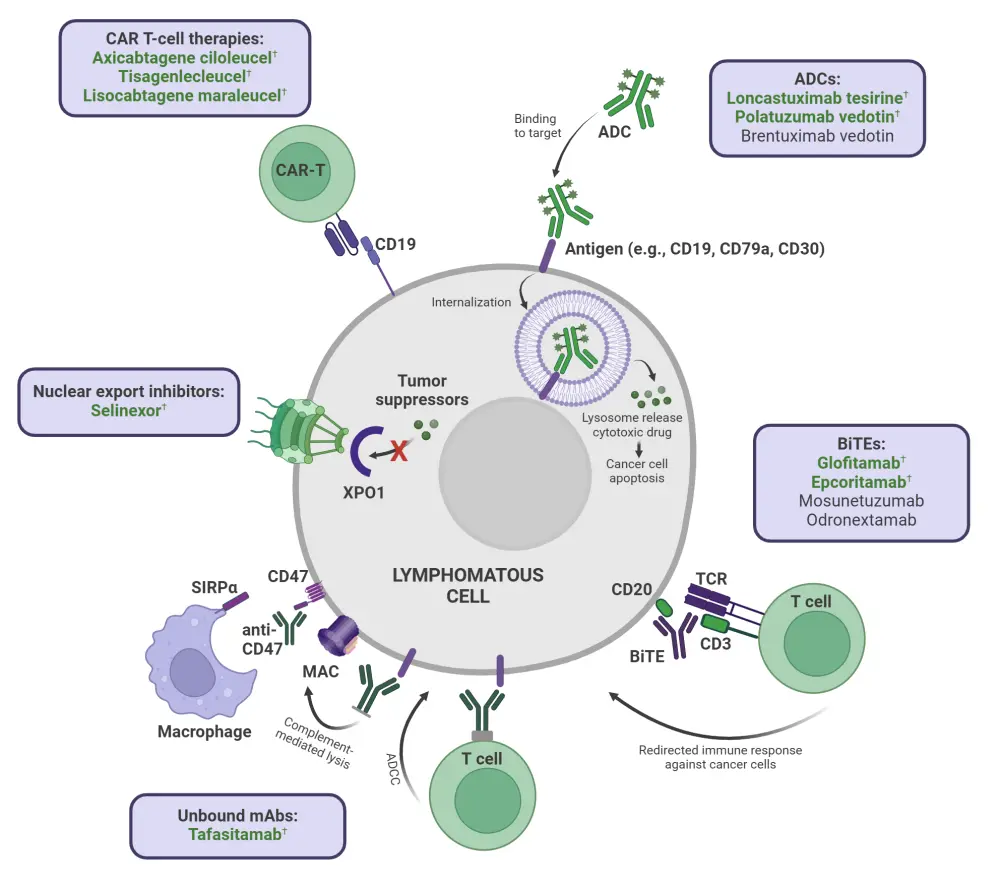
ADC, antibody–drug conjugate; ADCC, antibody-dependent cellular cytotoxicity; BiTE, bispecific T-cell engager; CAR, chimeric antigen receptor; mAb, monoclonal antibody; MAC, macrophage attack complex; SIRPα, signal regulatory protein α; XPO1, exportin 1.
*Adapted from Frontzek, et al.1 and Schipani, et al.2 Created with BioRender.com.
†Approved agents.
Approved therapies for R/R DLBCL
Over the last decade, approval of novel agents of various classes and mechanisms has dramatically changed the treatment landscape of R/R DLBCL. Table 1 presents an overview of the recently approved agents, along with their key trials and outcomes.
Table 1. Therapies approved by the FDA for R/R DLBCL since 2017*
| Agent | Year of initial FDA approval | FDA indication(s) | Key trials and outcomes |
| CAR T-cell therapies |
|||
| Axicabtagene ciloleucel (axi-cel)3–6 | 2017 | Adult patients with LBCL refractory to first-line chemoimmunotherapy or that relapses within 12 months of first-line chemoimmunotherapy. Adult patients with R/R LBCL after ≥2 lines of systemic therapy, including DLBCL not otherwise specified, primary mediastinal LBCL, high-grade B-cell lymphoma, and DLBCL arising from FL. |
Phase II ZUMA-1 (NCT02348216; N = 358)3: OR, 83% CR, 58% Phase III ZUMA-7 (NCT03391466; N = 180; axi-cel vs standard care)5: EFS, 8.3 months vs 2.0 months 24-mEFS, 41% vs 16% (p < 0.001) |
| Tisagenlecleucel (tisa-cel)3,6,7 | 2018 | Adult patients with R/R LBCL after ≥2 lines of systemic therapy, including DLBCL, high-grade B-cell lymphoma, and DLBCL arising from FL. | Phase II JULIET (NCT02445248; n = 93): OR, 52%3 CR, 40%3 12-mOS, 49%6 |
| Lisocabtagene maraleucel (liso-cel)3,8,9 | 2021 | Adult patients with LBCL, including DLBCL not otherwise specified (including DLBCL arising from indolent lymphoma), high-grade B-cell lymphoma, primary mediastinal LBCL, and FL Grade 3B, who have refractory disease to first-line chemoimmunotherapy or relapse within 12 months of first-line chemoimmunotherapy; refractory disease to first-line chemoimmunotherapy or relapse after first-line chemoimmunotherapy and ineligibility for HSCT due to comorbidities or age; or R/R after ≥2 lines of systemic therapy. |
Phase I TRANSCEND-NHL-001 (NCT02631044; N = 269)3: CR, 74% vs 43% mEFS, NR vs 2.4 months |
| Antibody–drug conjugates |
|||
| Polatuzumab vedotin (Pola)3,10 | 2019 | In combination with BR for adult patients with R/R DLBCL after ≥2 prior therapies. | Phase I/II GO29365 (NCT02257567; N = 80; Pola + BR vs BR): CR, 40% vs 17.5% |
| Loncastuximab tesirine11,12 | 2021 | Adult patients with R/R LBCL after ≥2 lines of systemic therapy, including DLBCL, DLBCL arising from low-grade lymphoma, and high-grade B-cell lymphoma. | Phase II LOTIS-2 (NCT03589469; N = 145): OR, 48.3% |
| Unbound monoclonal antibodies |
|||
| Tafasitamab3,13 | 2020 | In combination with lenalidomide for adult patients with R/R DLBCL, including DLBCL arising from low-grade lymphoma, and who are ineligible for auto-SCT. | Phase II L-MIND (NCT02399085; N = 81): OR, 61% CR, 43% |
| Bispecific antibodies | |||
| Epcoritamab14,15 | 2023 | R/R DLBCL, including DLBCL arising from indolent lymphoma and high-grade B-cell lymphoma after ≥2 lines of systemic therapy. | Phase I/II EPCORE NHL-1 (NCT03625037; ongoing; N = 128): OR, 82% CR, 62.5% |
| Glofitamab16 | 2023 | Adult patients with R/R DLBCL or LBCL arising from FL after ≥2 lines of systemic therapy. | Phase I/II NP30179 (NCT03075696; ongoing; N = 132); OR, 56% CR, 43% |
| Nuclear export inhibitors |
|||
| Selinexor6,17 | 2020 | Adult patients with R/R DLBCL, including DLBCL arising from FL after ≥2 lines of systemic therapy. | Phase IIb SADAL (NCT02227251; ongoing; N = 134): OR, 29% CR, 13% |
| ADC, antibody–drug conjugate; auto-SCT, autologous stem cell transplantation; axi-cel, axicabtagene ciloleucel; BR, bendamustine + rituximab; CAR, chimeric antigen receptor; CR, complete response; DLBCL, diffuse large B-cell lymphoma; EC, European Commission; FDA, U.S. Food and Drug Administration; FL, follicular lymphoma; LBCL, large B-cell lymphoma; liso-cel, lisocabtagene maraleucel; mEFS, median event-free survival; mOS, median overall survival; NR, not reached; OR, overall response; pola, polatuzumab vedotin; R/R, relapsed/refractory; SoC, standard of care. *Data from Brooks and Cami3, U.S. FDA4, Locke et al.5, Harris et al.6, U.S. FDA7,8, Business Wire9, U.S. FDA10,11, Cami et al.12, U.S. FDA13,14, Linton et al.15, U.S. FDA16,17. |
|||
Anti-CD19 CAR T-cell therapy
In 2017, axicabtagene ciloleucel (axi-cel) was the first CD19-directed CAR T-cell therapy to receive U.S. Food and Drug Administration (FDA) approval for the treatment of adult patients with certain types of large B-cell lymphoma (LBCL) who were R/R after ≥2 lines of systemic therapy, based on findings from the phase II ZUMA-1 trial.3,4 Axi-cel was further investigated in the phase III ZUMA-7 trial, which compared its efficacy to standard second-line treatment options, typically a platinum-based salvage combination chemotherapy regimen followed by HDT and ASCT.3,5 The positive results from the ZUMA-7 trial led to its FDA approval in 2022 as a second-line treatment for LBCL that is refractory to first-line chemoimmunotherapy or that relapses within 12 months of first-line chemoimmunotherapy.18
Tisagenlecleucel (tisa-cel) was approved by the FDA in 2018 for treatment of R/R LBCL after ≥2 lines of therapy, based on results from the phase II JULIET trial.7 It was later investigated as a second-line treatment for aggressive B-cell lymphoma in the phase III BELINDA trial (NCT03570892), but the study did not meet its primary endpoint.3,19
Lisocabtagene maraleucel (liso-cel) was approved by the FDA in 2021, based on results from the TRANSCEND-NHL-001 trial, for the treatment of adult patients with R/R LBCL after ≥2 lines of systemic therapy, including DLBCL not otherwise specified (including DLBCL arising from indolent lymphoma).8 Liso-cel was later assessed as a second-line treatment for patients with R/R DLBCL in the TRANSFORM study for those eligible for ASCT and in the PILOT study for those ineligible for ASCT.9 Based on findings from both the trials, the FDA approved liso-cel for the treatment of adult patients with DLBCL refractory to first-line treatment or with DLBCL that relapses within 12 months of first-line chemoimmunotherapy, as well as for patients with R/R DLBCL who are not eligible for HDT-ASCT due to comorbidities or age.9
Antibody–drug conjugates (ADCs)
Polatuzumab vedotin, a CD79b-directed ADC, received FDA approval in 2019, in combination with bendamustine and rituximab in ASCT-ineligible adult patients with R/R DLBCL after ≥2 prior therapies, based on results from the GO29365 study.10
Loncastuximab tesirine, a CD19-targeting ADC, received accelerated FDA and conditional European Union approval for R/R DLBCL after ≥2 lines of systemic therapy based on data from the phase II LOTIS-2 trial.11 It is currently being investigated in the phase III LOTIS-5 trial to compare its efficacy in combination with rituximab vs standard immunochemotherapy of rituximab + gemcitabine + oxaliplatin (R-GemOx) in R/R DLBCL (Table 2).20
Unbound monoclonal antibodies
Tafasitamab, a humanized anti-CD19 monoclonal antibody, in combination with lenalidomide, was granted accelerated approval by the FDA in 2020 as second-line treatment of R/R DLBCL in patients ineligible for HDT-ASCT,13 based on the results from the phase II L-MIND trial.
Watch our expert opinion interview with Lymphoma Hub Chair, Gilles Salles discussing the L-MIND trial results and how we might sequence the L-MIND regimen compared with CAR T-cell therapy.
Bispecific antibodies (bsAbs)
Epcoritamab and glofitamab, bsAbs targeting CD3 and CD20, were granted accelerated FDA approval in 2023 for R/R DLBCL following ≥2 lines of therapy.14,16
Odronextamab, a CD3- and CD20-targeting bsAb, received approval by the European Commission in 2024 for the treatment of adult patients with R/R follicular lymphoma (FL) or R/R DLBCL who progress after ≥2 lines of systemic therapy, including a CAR T-cell therapy.21 This was based on results from the ongoing phase I ELM-1 trial (NCT02290951) and the phase II ELM-2 trial (NCT03888105). Among 127 patients with R/R DLBCL in the ELM-2 trial, odronextamab was associated with an overall response rate of 52%, with 31% of patients achieving a complete response.21 Of note, odronextamab is not yet approved by the FDA for R/R DLBCL.
Watch our expert opinion interview with Won Kim discussing the ELM-2 trial results and clinical implications.
Nuclear export inhibitors
Selinexor, an oral exportin-1 inhibitor, received accelerated approval from the FDA in 2020 for the treatment of patients with R/R DLBCL after receiving ≥2 prior lines of therapy.3 The approval was based on the results from the phase IIb SADAL trial, which included patients both ineligible for transplant or those who relapsed following HDT-ASCT.3
Question 1 / 1
Which of the following agents has been approved by the European Commission for R/R DLBCL but is yet to be approved by the FDA?
A
Odronextamab
B
Brexucabtagene autoleucel
C
Tafasitamab
D
Mosunetuzumab
Key ongoing trials of the approved therapies for R/R DLBCL
Given the promising outcomes of approved novel therapies in third-line R/R settings, many are now being investigated in earlier lines (Table 2).
Table 2. Key ongoing phase III trials of previously approved agents for R/R DLBCL*
| Regimen | Trial | Trial status and completion year |
| Bispecific antibodies |
||
| Glofitamab + gemcitabine + oxaliplatin vs rituximab + gemcitabine + oxaliplatin22 | STARGLO; NCT04408638 | Active, not recruiting; 2025 |
| Epcoritamab + lenalidomide vs rituximab + gemcitabine + oxaliplatin23 | EPCORE DLBCL-4; NCT06508658 | Recruiting; 2028 |
| Antibody–drug conjugates |
||
| Loncastuximab tesirine + rituximab20 | LOTIS-5; NCT04384484 | Recruiting; 2028 |
| Polatuzumab vedotin-R-ICE vs R-ICE24 | Pola-R-ICE; NCT04833114 | Recruiting; 2025 |
| Tafasitamab + lenalidomide25 | firmMIND; NCT05429268 | Recruiting; 2026 |
| Nuclear export inhibitors |
||
| R-GDP ± selinexor26 | NCT04442022 | Recruiting; 2025 |
| DLBCL, diffuse large B-cell lymphoma; R-GDP, rituximab + gemcitabine + dexamethasone + platinum; R-ICE, rituximab + ifosfamide + carboplatin + etoposide; R/R, relapsed/refractory. *Data from ClinicalTrials.gov20,22–26. |
||
Currently, findings from the STARGLO and Pola-R-ICE trials are available and these are summarized below.
STARGLO is an ongoing phase III trial evaluating the efficacy and safety of glofitamab + gemcitabine + oxaliplatin (Glofit-GemOx) vs rituximab (R)-GemOx as second-line treatment for ASCT-ineligible patients with R/R DLBCL.27 At a median follow-up of 20.7 months, the primary endpoint was met with significant improvement in OS with Glofit-GemOx vs R-GemOx (median OS, 25.5 months vs 12.9 months; hazard ratio, 0.62; 95% confidence interval, 0.43–0.88; p = 0.0064; Figure 2).27 The safety profile was consistent with known adverse events of individual agents (Figure 3).27 Based on the encouraging results, the FDA accepted a supplemental biologics license application for Glofit-GemOx on December 5, 2024, for the treatment of patients with R/R DLBCL who have received ≥1 prior line of therapy and are ineligible for ASCT.28 The approval decision is expected by July 2025.28 This off-the-shelf regimen could be a potential fixed-duration treatment option for patients with high-risk of disease progression.28
Figure 2. Efficacy of Glofit-GemOx vs R-GemOx: Results from the STARGLO trial*
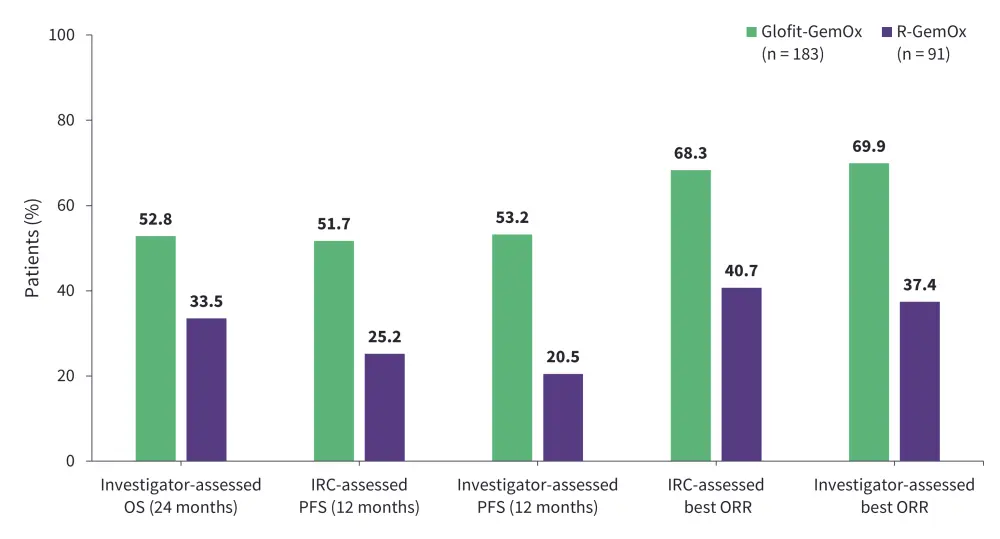
GemOx, gemcitabine + oxaliplatin; Glofit, glofitamab; IRC, independent review committee; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; R, rituximab.
*Data from Abramson, et al.27
Figure 3. Safety profile of Glofit-GemOx vs R-GemOx: Results from the STARGLO trial*
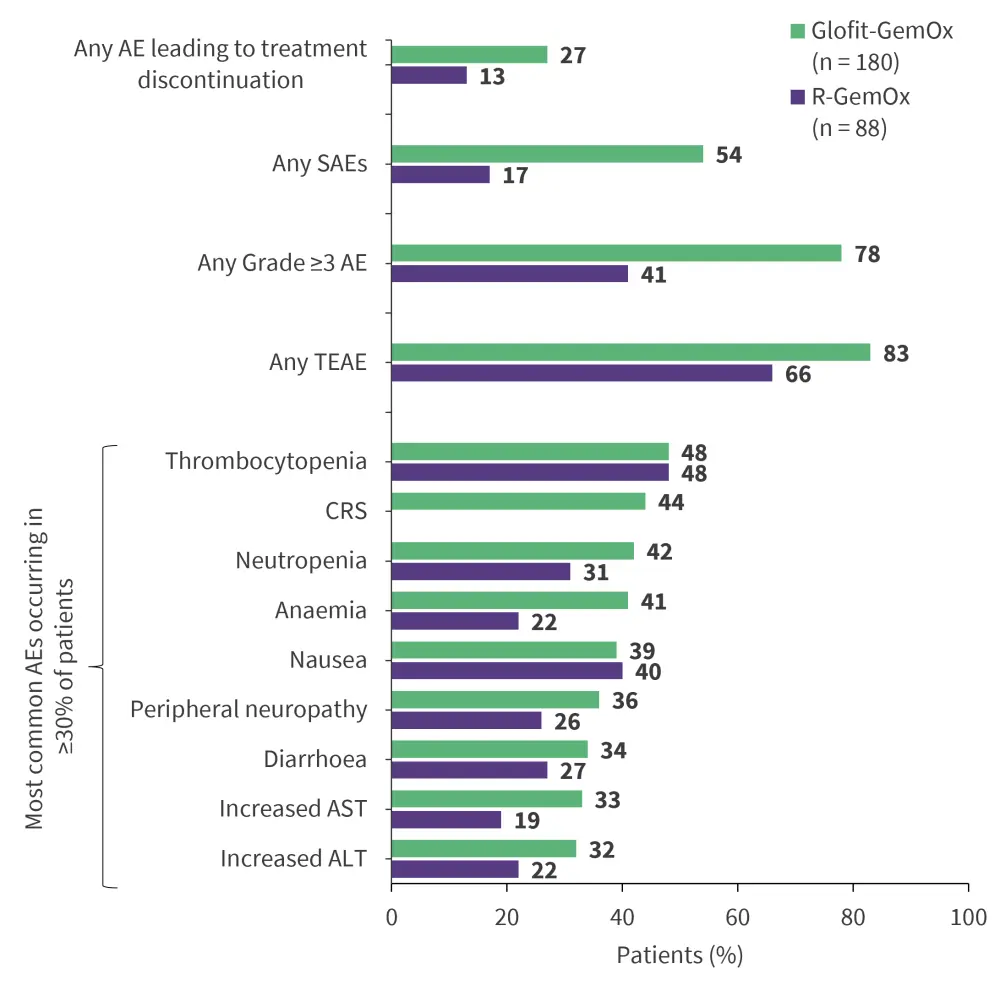
AE, adverse event; ALT, alanine transaminase; AST, aspartate transferase; CRS, cytokine release syndrome; GemOx, gemcitabine + oxaliplatin; Glofit, glofitamab; R, rituximab; SAE, serious AE; TEAE, treatment-emergent AE.
*Data from Abramson, et al.27
Question 1 / 1
In the phase III STARGLO trial, what was the median OS with Glofit-GemOx at a median follow-up of 20.7 months?
A
19.2 months
B
27.1 months
C
25.5 months
D
22 months
Pola-R-ICE is an ongoing phase III trial evaluating the safety and efficacy of polatuzumab in combination with rituximab + ifosfamide + carboplatin + etoposide (Pola-R-ICE) as second-line treatment of R/R DLBCL followed by ASCT.29 Among 41 enrolled patients, the primary endpoint of objective response was achieved by 92% of 38 evaluable patients (Figure 4).29 The most common treatment-emergent adverse events related to Pola-R-ICE were anemia (78%), nausea (70%), thrombocytopenia (70%), leukopenia (50%), fatigue (48%), neutropenia (48%), lymphopenia (38%), constipation (35%), hypertension (30%), and hypophosphatemia (28%).29 This regimen shows potential as a salvage therapy for transplant-eligible patients with R/R DLBCL, while longer follow-up results in a larger patient population are awaited.
Figure 4. Efficacy of Pola-R-ICE vs R-ICE: Results from the Pola-R-ICE trial*
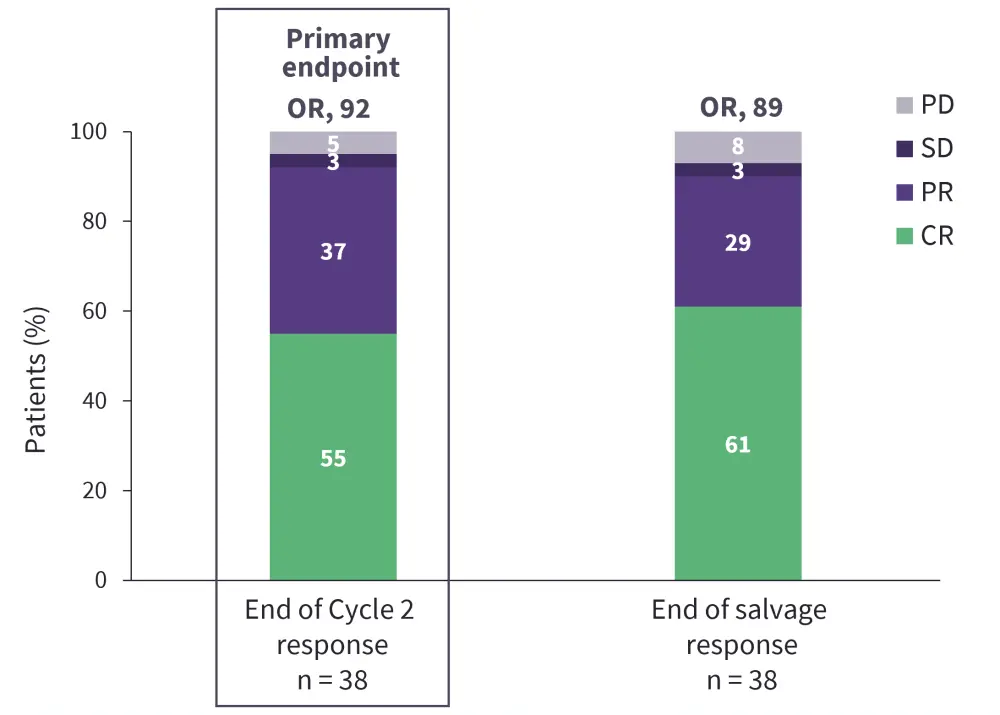
CR, complete response; OR, objective response; PD, progressive disease; Pola-R-ICE, polatuzumab + rituximab + ifosfamide + carboplatin + etoposide; PR, partial response; SD, stable disease.
*Data from Herrera, et al.29
To read more about second-line treatments for DLBCL, see our previously published article.
Novel therapies in development for R/R DLBCL
A number of novel therapies are also in development for R/R DLBCL (Table 3). Available data for these therapies is explored below.
Table 3. Key ongoing trials of novel agents for R/R DLBCL*
| Regimen | Class | Trial | Trial status and completion year |
| Brentuximab vedotin30 | ADC | Phase III ECHELON-3 (NCT04404283) | Active, not recruiting; 2027 |
| Mosunetuzumab31,32 |
bsAb |
Phase Ib/II (NCT03671018) | Active, not recruiting; 2025 |
| Phase III SUNMO (NCT05171647) | Active, not recruiting; 2027 | ||
| Maplirpacept (PF-07901801), tafasitamab, and lenalidomide33 | Anti-CD47 molecule | Phase I/II (NCT05626322) | Active, not recruiting; 2025 |
| TTI-622 + pembrolizumab34 | Fusion protein | Phase II (NCT05507541) | Recruiting; 2027 |
| DZD858635,36 | LYN/BTK dual inhibitor | Phase II TAI-SHAN9 (NCT06539195) | Recruiting; 2028 |
| Golcadomide + rituximab37,38 | Cereblon E3 ligase modulator | Phase II (NCT06271057) | Recruiting; 2027 |
| ADC, antibody–drug conjugate; bsAb, bispecific antibody; DLBCL, diffuse large B-cell lymphoma; R/R, relapsed/refractory. *Data from ClinicalTrials.gov30–35, Song et al.36, ClinicalTrials.gov37, Chaver et al.38. |
|||
Brentuximab vedotin
Brentuximab vedotin (BV) is a CD30-directed ADC to monomethyl auristatin E, a microtubule-disrupting agent, approved for the treatment of classical Hodgkin lymphoma and anaplastic large cell lymphoma.3,39 Its safety and efficacy has been demonstrated in phase I (NCT02086604) and phase II (NCT01421667) trials in patients with R/R DLBCL who have received ≥1 prior line of therapy. BV is currently being investigated in the ongoing phase III ECHELON-3 trial (NCT04404283) in combination with lenalidomide + rituximab for the treatment of patients with R/R DLBCL who have received ≥2 prior lines of therapy.40 Interim analysis results revealed that BV + lenalidomide + rituximab combination met its primary endpoint by reducing the risk of death by 37% compared with placebo + lenalidomide + rituximab (Figure 5).40 Further, the overall response rate was significantly higher in the BV arm compared with the placebo arm, irrespective of CD30 expression (Figure 6).40 The safety profile was acceptable, with no new adverse events observed (Figure 7).40 The triplet combination showed potential to address an unmet clinical need for patients with R/R DLBCL, particularly those who are ineligible for or relapse following ASCT or CAR T-cell therapy.40 Additionally, the results are promising as the study included heavily pre-treated patients, with a median of three prior lines of systemic therapies and ~30% patients having received prior CAR-T therapy.41 Furthermore, a subset analysis of the ECHELON-3 trial in patients with R/R DLBCL aged ≥65 years (n = 155) and ≥75 years (n = 86) was performed.42 The primary endpoint of median overall survival was significantly improved with BV + lenalidomide + rituximab vs placebo + lenalidomide + rituximab in both the ≥65 years group (15.9 months vs 8.5 months; p = 0.0043) and ≥75 years group (21.5 months vs 8.5 months; p = 0.0189), suggesting its effectiveness in older patients with R/R DLBCL.42 Though not yet approved by the FDA for this indication, BV is listed as a therapeutic option by the National Comprehensive Cancer Network (NCCN) guidelines for CD30-positive R/R DLBCL.43
Figure 5. Interim analysis results from the ECHELON-3 trial: Key primary and secondary endpoints*
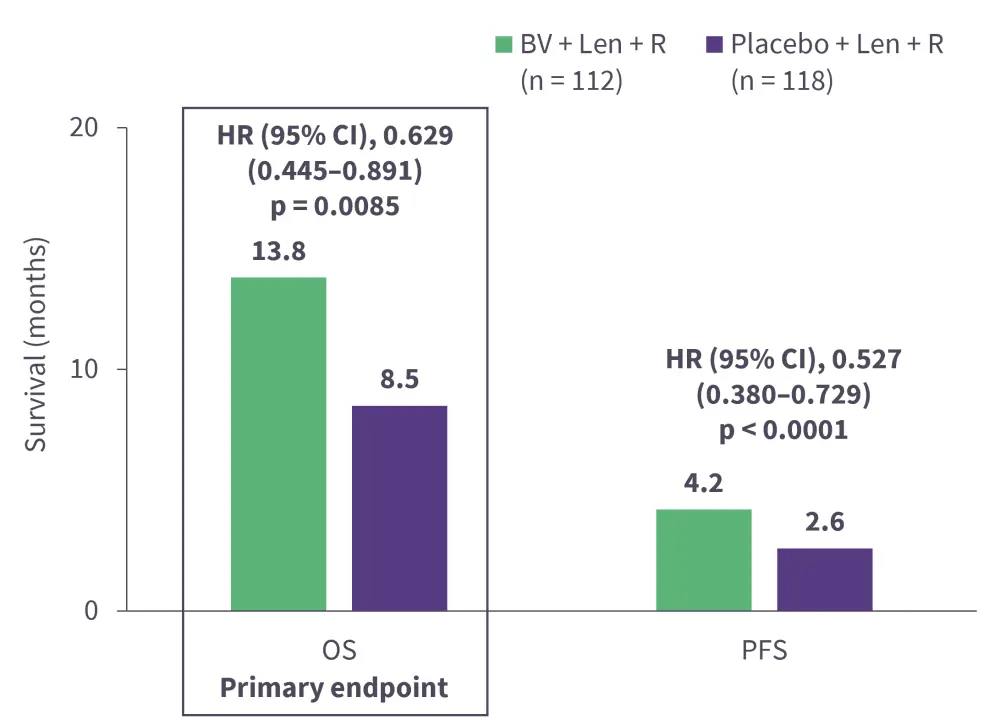
CI, confidence interval; HR, hazard ratio; Len, lenalidomide; OS, overall survival; PFS, progression-free survival; R, rituximab.
*Data from Kim et al.40
Figure 6. Interim analysis results from the ECHELON-3 trial: ORR*
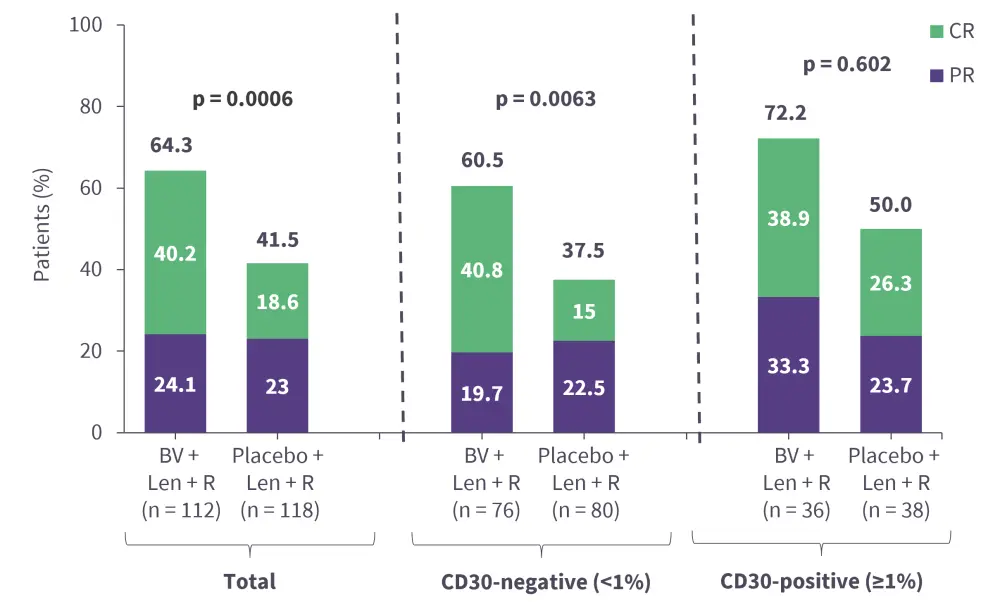
CR, complete response; Len, lenalidomide; ORR, overall response rate; PR, partial response; R, rituximab.
*Data from Kim et al.40
Figure 7. Interim analysis results from the ECHELON-3 trial: Key safety findings*
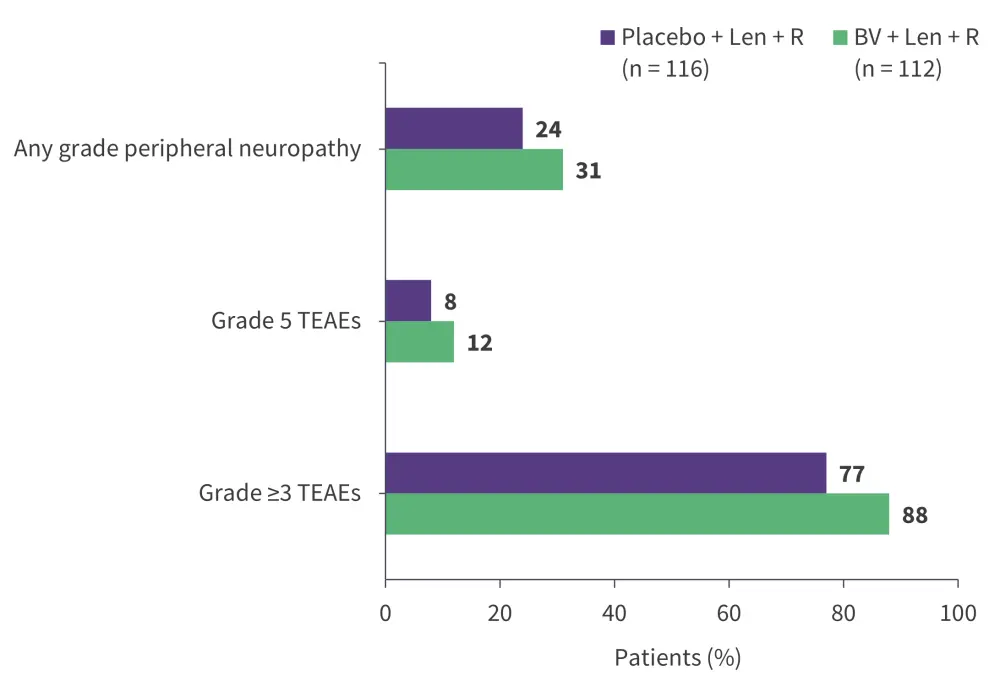
Len, lenalidomide; R, rituximab; TEAE, treatment-emergent adverse event.
*Data from Kim et al.40
Question 1 / 1
In a subgroup analyses of the phase III ECHELON-3 study, what was the median overall survival with BV + lenalidomide + rituximab in patients with R/R DLBCL aged ≥75 years?
A
21.5 months
B
18.2 months
C
13.8 months
D
15.9 months
Mosunetuzumab
Mosunetuzumab, a CD20 x CD3 T cell-engaging bsAb, is an FDA-approved treatment for R/R FL after ≥2 lines of systemic therapy,44 and is currently under investigation for R/R DLBCL. In a phase Ib/II trial (NCT03671018),31 subcutaneous administration of mosunetuzumab + polatuzumab vedotin (M-Pola) showed improved efficacy (Figure 8) and safety (Figure 9) compared with intravenous administration of rituximab + Pola (R-Pola) in patients with R/R DLBCL after ≥2 lines of prior systemic therapy.45 While longer follow-up is needed, the findings support further investigation of M-Pola in transplant- ineligible patients with LBCL in the phase III SUNMO trial (NCT05171647).32,45
Figure 8. Efficacy of M-Pola vs R-Pola*
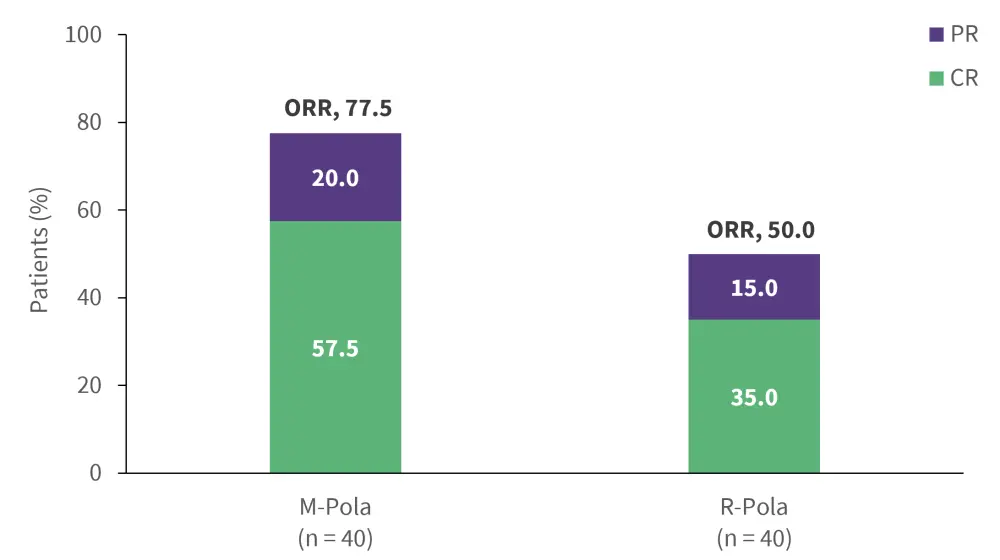
CR, complete response; M, mosunetuzumab; ORR, overall response rate; Pola, polatuzumab vedotin; PR, partial response; R, rituximab.
*Data from Chavez et al.45
Figure 9. Safety profile of M-Pola vs R-Pola*
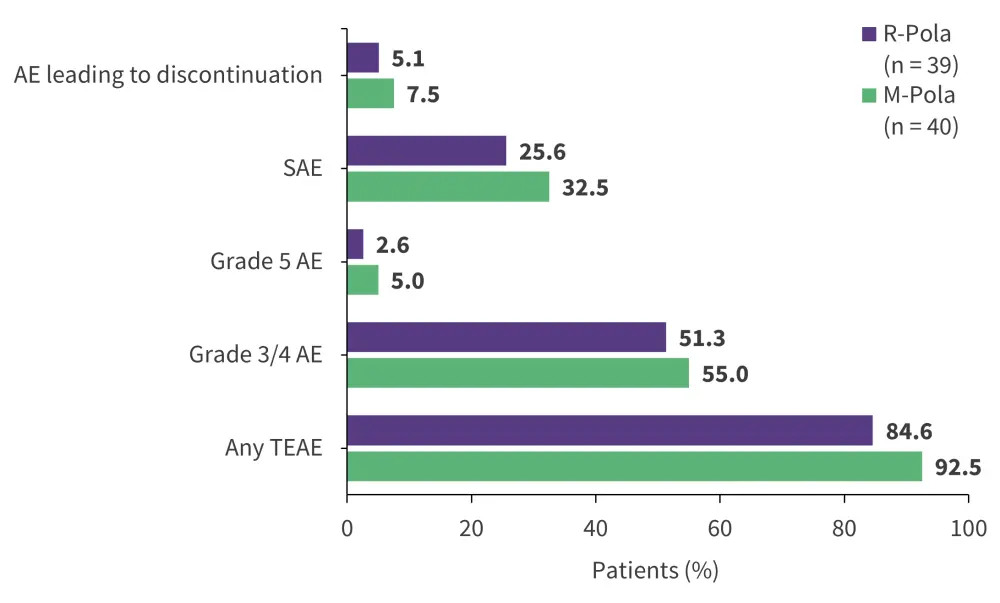
AE, adverse event; M, mosunetuzumab; Pola, polatuzumab vedotin; R, rituximab; SAE, serious AE; TEAE, treatment-emergent AE.
*Data from Chavez et al.45
Question 1 / 1
What is the primary cellular target of mosunetuzumab?
A
CD19 and CD20
B
CD20 and CD3
C
CD3
D
CD20
Conclusion
Novel agents are revolutionizing the treatment landscape for R/R DLBCL, providing new treatment pathways following the failure of cytotoxic chemoimmunotherapy. Landmark advancements include the FDA approval of CAR T-cell therapies (axi-cel, tisa-cel, liso-cel), ADCs (polatuzumab vedotin and loncastuximab tesirine), bsAbs (epcoritamab and glofitamab), and nuclear export inhibitors (selinexor) for R/R DLBCL after ≥2 prior lines of systemic therapy, and thereby increasing the treatment options for heavily pre-treated patients with R/R disease. Following promising results, a number of agents have been further approved in earlier lines. Several other approved agents, including glofitamab, epcoritamab, and polatuzumab, are currently being investigated for their potential in second-line treatment. Recently, the bispecific antibody odronextamab received European Commission approval and is awaiting FDA approval, while brentuximab vedotin (an ADC) and mosunetuzumab (a bsAb) are emerging as promising treatment options for R/R DLBCL. Future directions include targeting novel pathways, developing synergistic combination regimens, and adopting personalized approaches informed by the disease's molecular characteristics.
Your opinion matters
After reading this educational resource, I commit to reviewing the latest advancements in the field for the management of patients with R/R DLBCL.
This educational resource was independently supported by Pfizer. All content was developed by SES in collaboration with an expert steering committee; funders were allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



