All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Glofit-GemOx vs R-GemOx in patients with R/R DLBCL: Results from the STARGLO trial
Featured:
Do you know... For which patient population may Glofit-GemOx be the most appropriate treatment option?
During the Lymphoma Hub Steering Committee Meeting on October 6, 2025, key opinion leaders met to discuss the results from the phase III STARGLO trial (NCT04408638) of glofitamab (Glofit) + gemcitabine + oxaliplatin (GemOx) vs rituximab (R)-GemOx in patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL). The discussion was preceded by a presentation by Gareth Gregory, chaired by Gilles Salles, and featured Ulrich Jäger, Francesc Bosch, Michael Dickinson, Astrid Pavlovsky, Marek Trněný, Alison Moskowitz, and Stefano Luminari.
Glofit-GemOx vs R-GemOx in patients with R/R DLBCL: Results from the STARGLO trial
Glofit-GemOx vs R-GemOx in patients with R/R DLBCL: Results from the STARGLO trial
Presentation
Gregory presented an overview of the STARGLO trial, efficacy and safety data, and key takeaways.
Background and study design
Patients with R/R DLBCL generally have a poor prognosis, particularly patients who are ineligible for cellular therapies or who relapse after second-line treatment.1 For patients with primary refractory disease following first-line treatment, or those who relapse within 12 months, chimeric antigen receptor (CAR) T-cell therapies have markedly improved survival; however, CAR T-cell therapies are not uniformly available.1 Glofitamab, an off-the-shelf T-cell engaging CD20/CD3 bispecific antibody, has demonstrated durable responses as a fixed-duration monotherapy in patients with R/R DLBCL after ≥2 prior lines of therapy.2
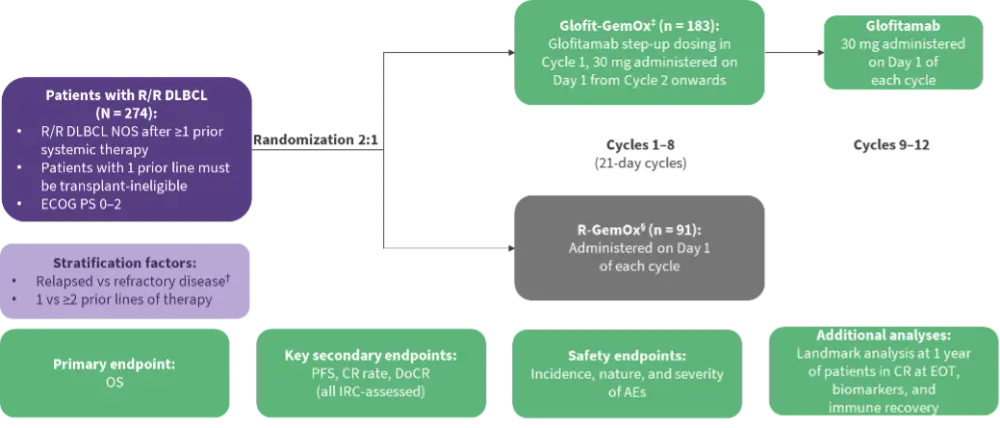
The 2:1 randomized, open-label, multicenter, phase III STARGLO trial assessed the efficacy and safety of Glofit-GemOx vs R-GemOx in 274 patients with ≥1 prior line of systemic therapy (Figure 1).1 In the primary analysis, Glofit-GemOx showed a significant benefit in overall survival (OS), progression-free survival (PFS), and complete response (CR) rate vs R‑GemOx in transplant-ineligible patients with R/R DLBCL.1 Gregory presented updated 2-year follow-up results.
Figure 1. STARGLO study design*
Efficacy and safety3
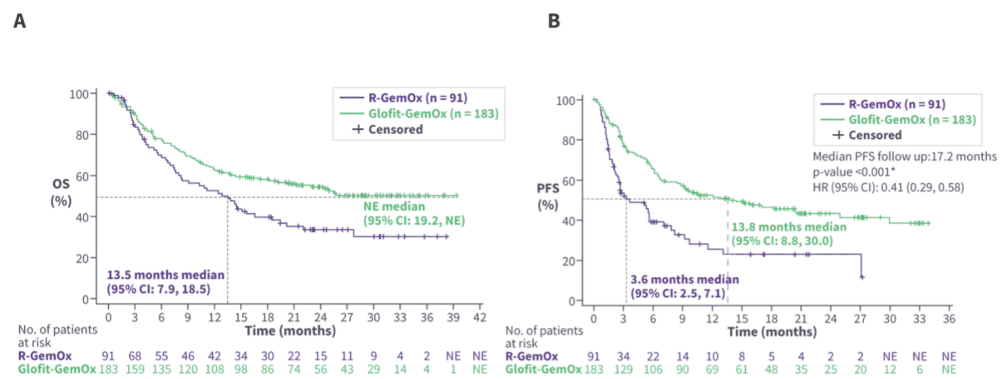
After 2 years of follow-up, Glofit-GemOx continued to show an OS and PFS benefit vs R-GemOx (Figure 2). Patients treated with Glofit-GemOx had a higher overall response rate (68.3% vs 40.7%) and CR rate (58.5% vs 25.3%) vs R-GemOx. The median duration of CR was not evaluable in the Glofit-GemOx arm compared with 24.2 months in the R-GemOx arm. The ongoing CR rate was 42.1% and 17.6% in the Glofit-GemOx arm and the R-GemOx arm, respectively. The landmark analysis at the end of treatment (EOT) in the Glofit-GemOx arm showed that over 80% of patients with a CR at the EOT remained progression-free and alive 12 months after EOT. Biomarker analyses demonstrated that immune recovery at 18–24 months was comparable between treatment arms.
Figure 2. Glofit-GemOx vs R-GemOx A OS and B PFS in the STARGLO trial*
The safety profile was consistent with the known safety profile of the individual study drugs, with no new safety signals with this additional follow-up (Table 1). Cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) were predominantly low grade. A limited number of Grade 5 adverse events (AEs) related to COVID-19 were observed early in the study. Following a protocol amendment excluding patients who tested positive for COVID-19 within 3 months prior to screening or who tested positive on study, no further Grade 5 events occurred.
Table 1. Safety profile in the STARGLO trial*
| n (%), unless otherwise stated | R-GemOx n = 88 | Glofit-GemOx (Glofit-exposed) n = 172 |
| Number of infusions, median (range) | 4 (1–8) | 12 (1–14) |
| Serious AEs | 15 (17.0) | 90 (52.3) |
| Grade ≥3 AEs | 36 (40.9) | 132 (76.7) |
| Grade 5 AEs | 4 (4.5) | 12 (7.0) |
| AE leading to any treatment discontinuation | 11 (12.5) | 44 (25.6) |
| CRS (any grade) | NA | 77 (44.8) |
| Grade 3–4† | NA | 4 (2.3) |
| ICANS (any grade) | NA | 4 (2.3) |
| Grade 3–4† | NA | 1 (0.6) |
| Infections (any grade) | 26 (29.5) | 95 (55.2) |
| Grade 3–4 | 8 (9.1) | 29 (16.9) |
| Grade 5 | 3 (3.4) | 6 (3.5)‡ |
*Adapted from Abramson JS.3 | ||
Key takeaways
After 2 years of follow-up, treatment with Glofit-GemOx demonstrated durable improvements in survival and response outcomes compared with R-GemOx in the STARGLO trial. Evidence of immune recovery was observed at 18–24 months following the fixed-duration treatment. The combination of Glofit-GemOx demonstrated a manageable safety profile consistent with the known effects of the individual agents, with no new safety signals. These findings support the use of Glofit-GemOx as an effective, fixed-duration, off-the-shelf treatment option for transplant-ineligible patients with R/R DLBCL.
Discussion: Key points
- Glofit-GemOx is a valuable addition to the treatment landscape for patients with R/R DLBCL, particularly for those who are ineligible for or unable to access CAR T-cell therapy.
- Immune recovery following fixed-duration Glofit-GemOx included B-cell reconstitution, while data on T-cell recovery are yet to be presented.
- Reports of atypical viral reactivation highlight the need for optimized monitoring and prophylactic strategies during and after treatment.
- Prior treatment exposure, cytopenias, patient preference, and regional access to CAR T-cell therapies impact patient selection for Glofit-GemOx.
- Glofit-GemOx is a suitable third-line therapy, or a second-line therapy for patients who are unable to access CAR T-cell therapies.
- Glofit-GemOx is feasible and deliverable in routine clinical practice, and adherence to the STARGLO regimen is recommended.
- Glofit-GemOx may have potential as a bridge to CAR T-cell therapy, particularly for high-risk patients, given the manageable toxicity and convenient dosing schedule, although this requires further investigation.
- Regional differences in access to and reimbursement for Glofit-GemOx currently limit availability, with wider adoption expected with further follow-up data and funding approval.
- Longer follow-up data from the STARGLO trial are needed to clarify the durability of benefit and the positioning of Glofit-GemOx within treatment sequencing for R/R DLBCL.
This educational resource is independently supported by Roche. All content was developed by SES in collaboration with an expert steering committee. Funders were allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Ulrich Jäger
Ulrich Jäger Michael Dickinson
Michael Dickinson Stefano Luminari
Stefano Luminari Marek Trněný
Marek Trněný Gilles Salles
Gilles Salles Francesc Bosch
Francesc Bosch Astrid Pavlovsky
Astrid Pavlovsky Alison Moskowitz
Alison Moskowitz

