All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
SYMPHONY-1: A phase Ib/III trial of TAZ+Len+R in R/R FL
⭐Lymphoma Hub spotlight | SYMPHONY-1: A phase Ib/III trial of TAZ+Len+R in R/R FL⭐
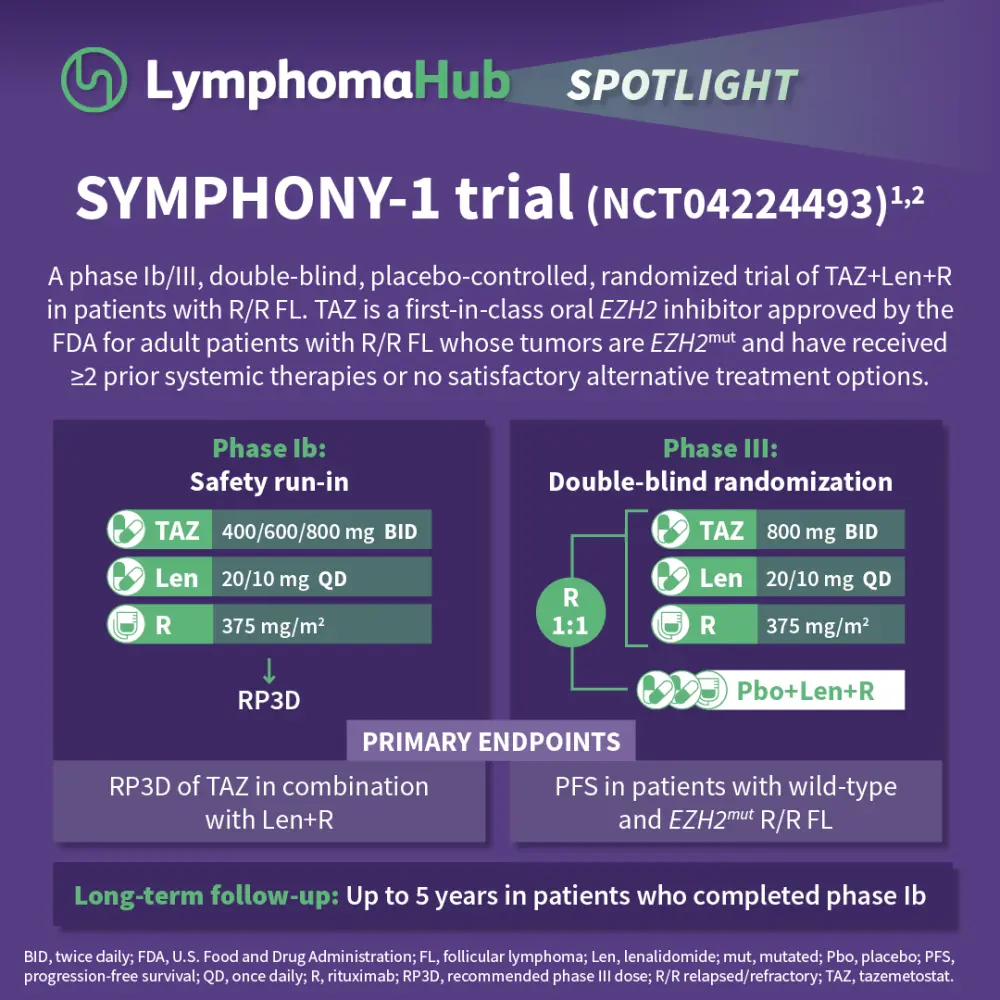


Tazemetostat is a first-in-class oral EZH2 inhibitor approved by the U.S. Food and Drug Administration (FDA) for adult patients with relapsed/refractory (R/R) follicular lymphoma (FL) whose tumors are positive for an EZH2mut and have received ≥2 prior systemic therapies or who have no satisfactory alternative treatment options.1
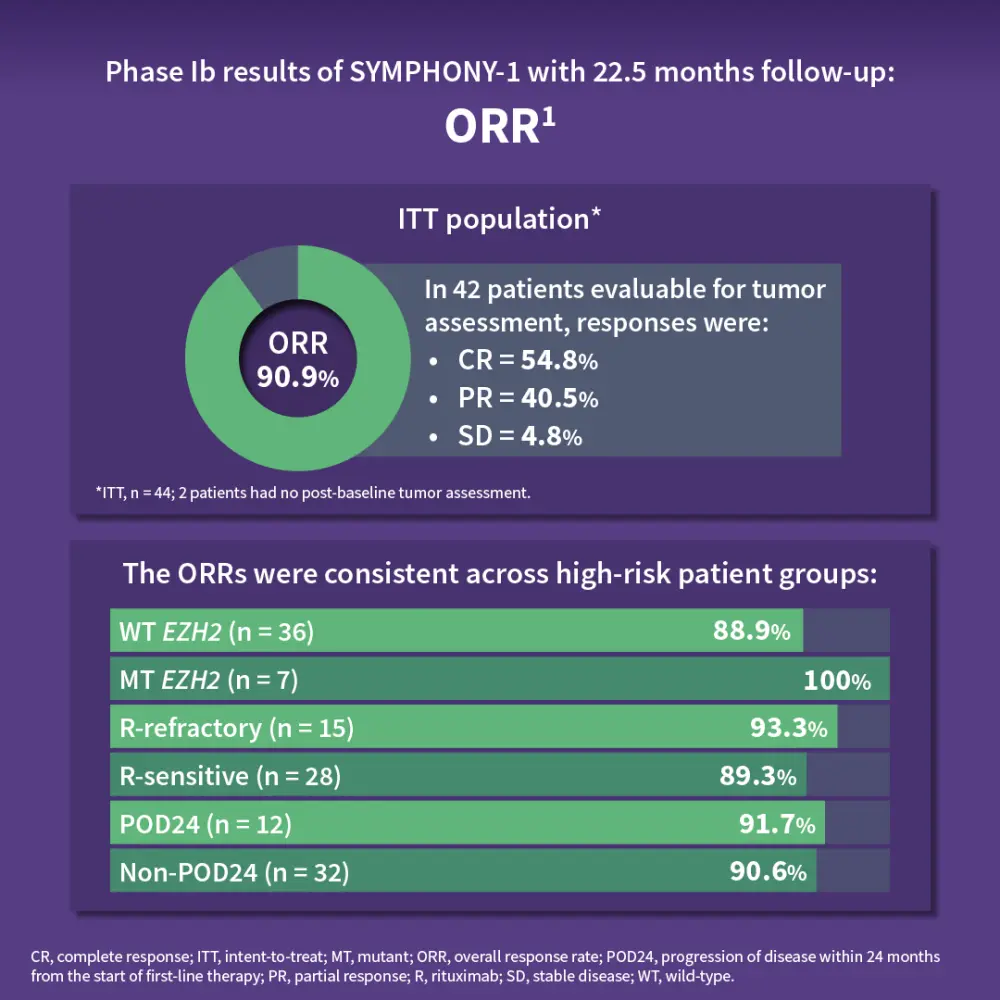
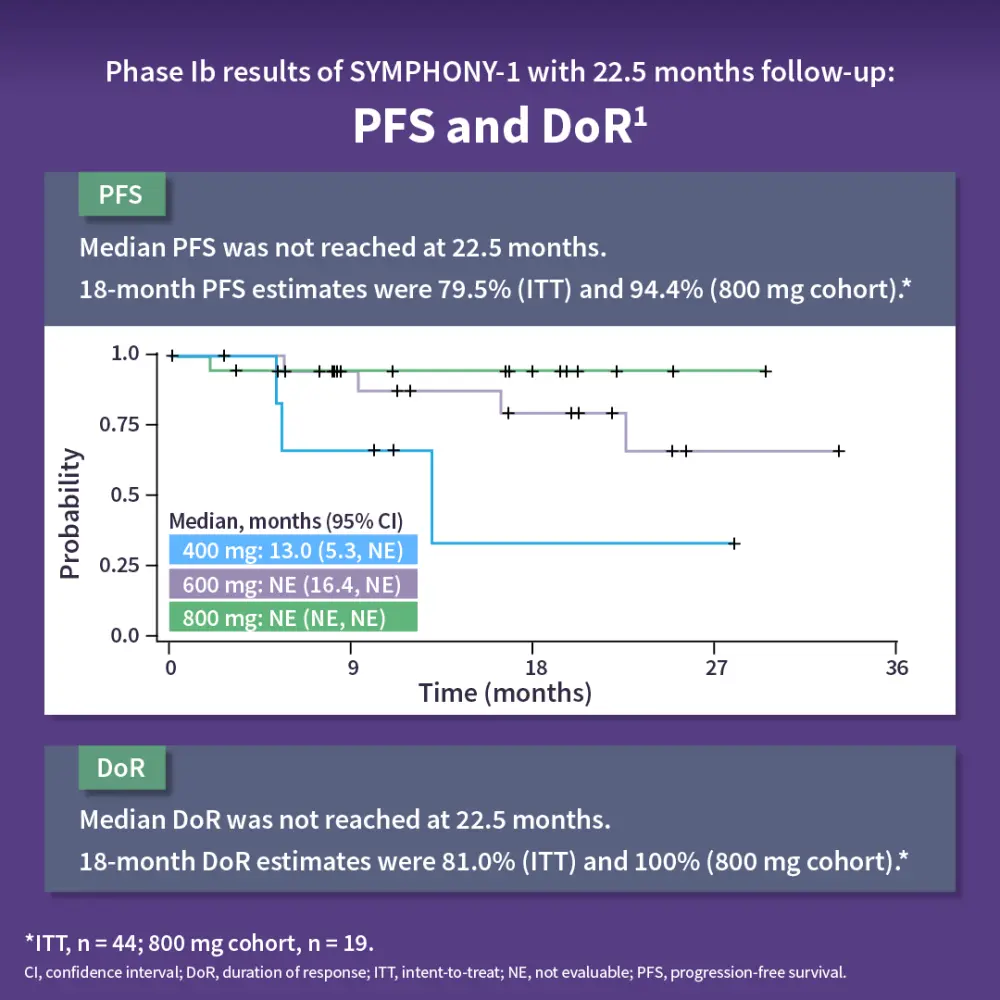
The SYMPHONY-1 trial is a phase Ib/III, double blind, placebo-controlled study of tazemetostat with lenalidomide and rituximab in R/R FL. The primary endpoints were to determine recommended phase III dose and progression-free survival in patients with wild-type and EZH2mut R/R FL.1,2 Results showed that the combination was well tolerated, with an 800 mg twice daily dose of tazemetostat recommended for phase III. At 22.5 months, the overall response rate was 90.9%, with a complete response rate of 54.8%. The ORR was consistent across subgroups, including patients with wild-type or EZH2mut and those who were rituximab refractory or sensitive. The median progression-free survival and duration of response were not reached, with 18-month PFS estimates of 79.5% (intent-to-treat) and 94.4% (800 mg cohort). The safety profile was consistent with prior data, with neutropenia as the most common severe adverse event. The phase III trial enrolling ~500 patients is ongoing, with primary completion expected in early 2026.1
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content




