All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Novel therapies for relapsed/refractory follicular lymphoma: Key updates from ICML 2023
Although the survival outcomes for patients with follicular lymphoma (FL) have advanced, there is an unmet need for improved tumor control and extended progression-free survival (PFS) in this population, due to variability FL management.
During the 17th International Conference on Malignant Lymphoma (17-ICML), updates from five key trials of novel therapies for the treatment of relapsed/refractory (R/R) FL were presented. Here, we are pleased to summarize these updates; Zinzani presented an updated analysis on the efficacy of zanubrutinib plus obinutuzumab,1 Novelli presented the efficacy and safety of odronextamab,2 Sehn presented an updated analysis of a phase II study of mosunetuzumab,3 Belada presented pooled analyses from the ongoing EPCORE NHL-2 trial,4 and Morschhauser presented data from the TRANSCEND study.5
Zanubrutinib in combination with obinutuzumab1
ROSEWOOD (NCT03332017) is an open-label, randomized, global phase II trial investigating zanubrutinib plus obinutuzumab versus obinutuzumab monotherapy in patients aged ≥18 years with R/R FL who had received ≥2 prior lines of therapy. The primary endpoint was overall response rate (ORR) assessed by an independent review committee (IRC) according to Lugano 2014 criteria. Key secondary endpoints included duration of response (DoR), PFS, overall survival (OS), time to next treatment, and safety.
- Overall, 217 patients were randomized, 145 patients to the zanubrutinib plus obinutuzumab and 72 to the obinutuzumab monotherapy arm with a median age of 63 and 65.5 years, respectively.
- The median number of prior therapies was three, with 98.6% of patients receiving prior chemotherapy.
- A FL International Prognostic Index (FLIPI) score of ≥3 at screening was observed in 53.1% and 51.4% of patients in zanubrutinib plus obinutuzumab and obinutuzumab monotherapy arm, respectively.
- At a median follow-up of 20.2 months, ORR was 69% vs 45.8% in the zanubrutinib plus obinutuzumab and obinutuzumab monotherapy arm, respectively (p = 0.0012).
- Complete response rate (CRR) was 39.3% vs 19.4% in the zanubrutinib plus obinutuzumab and obinutuzumab monotherapy arm, respectively (p = 0.0035).
- The 18-month DoR rate was 69.3% vs 41.9%, median PFS was 28 vs 10.4 months (p = 0.007) and median OS at 24 months was 34.6% vs not reached in the zanubrutinib plus obinutuzumab versus obinutuzumab arm.
- Median time to next treatment was not evaluable in the zanubrutinib plus obinutuzumab arm versus 12.2 months in the obinutuzumab arm.
- Grade ≥3 non-hematologic treatment-emergent adverse events (TEAEs) were recorded in less than 10% of patients in both arms, with pneumonia being the most commonly reported TEAE (Figure 1).
Figure 1. Grade ≥3 non-hematologic TEAEs*
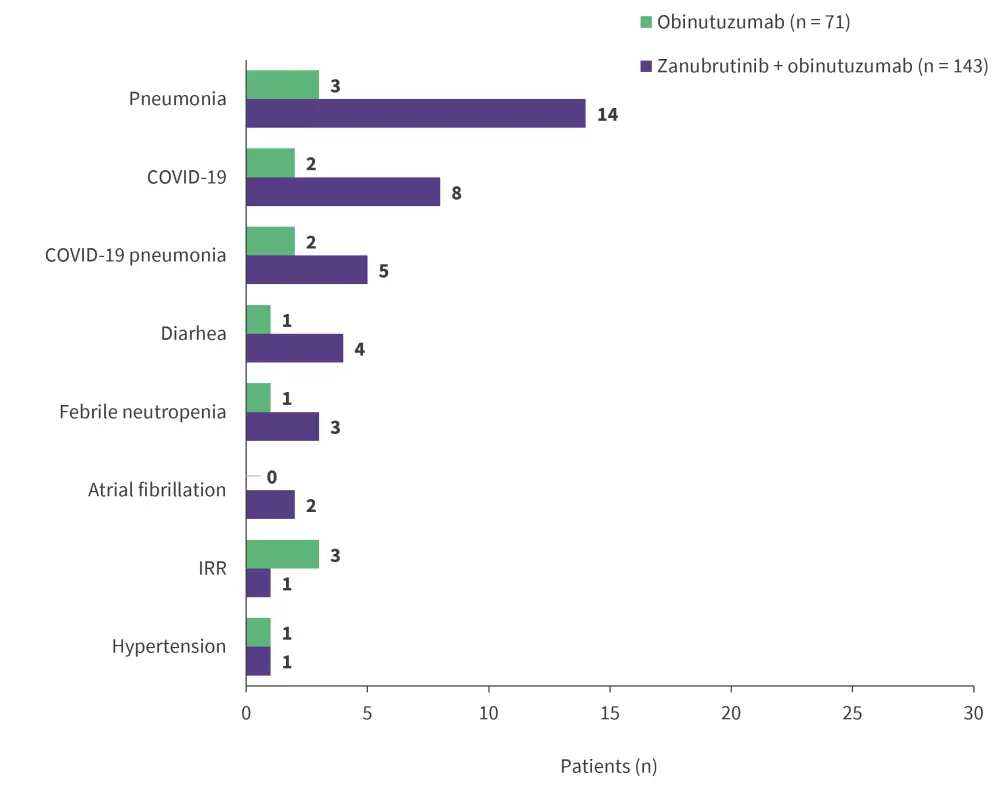
TEAE, treatment-emergent adverse event; IRR, infusion-related reaction.
*Adapted from Zinzani.1
Presenter’s conclusions
This follow-up analysis demonstrated the efficacy and manageable safety profile of zanubrutinib plus obinutuzumab in heavily pretreated patients with R/R FL, potentially representing a novel combination therapy for this patient population. The phase III MAHOGANY study (NCT05100862) is currently investigating this combination in patients who have received ≥1 lines of systemic therapy.
Odronextamab monotherapy2
ELM-2 (NCT03888105) is an open-label multicenter, multi-cohort, phase II study investigating odronextamab monotherapy with step-up dosing in Cycle 1. The FL cohort for this analysis comprised patients with R/R FL Grade 1–3a who were heavily pretreated (≥2 prior lines of therapy). The primary endpoint was ORR assessed by IRC. Key secondary endpoints included PFS, OS, and safety.
- Overall, 131 patients were treated with a median age of 61 years, 53% were male, 71% were refractory to the last therapy, and 48% had experienced disease progression within two years.
- At a median follow-up of 22.4 months, ORR and CRR were 82% and 75%, respectively.
- Median PFS was 20.2 months with 12- and 18-month PFS rates of 64% and 55.3%, respectively.
- Median OS was not reached with 12- and 18-month OS rates of 86.1% and 76.3%, respectively.
- Grade ≥3 TEAEs occurred in 77.9% of patients and Grade 5 TEAEs occurred in 13% of patients.
- Grade 5 treatment-related AEs (TRAEs) included pneumonia, progressive multifocal leukoencephalopathy, and systemic mycosis (one patient each).
- Serious TEAEs and serious TRAEs were reported in 61.8% and 40.5% of patients, respectively.
- The most common TEAEs included cytokine release syndrome (CRS; 56.5%), neutropenia (39.7%), pyrexia (31.3%), and anemia (29.8%).
- All CRS events were resolved, with a median time to resolution of 2 days (range 1–51 days).
- No immune effector cell-associated neurotoxicity syndrome or tumor lysis syndrome were reported.
Presenter’s conclusions
The FL cohort analysis demonstrates the efficacy of odronextamab, with deep and durable responses. The optimized step-up regimen of odronextamab was well-tolerated, highlighting the potential of odronextamab for the treatment of heavily pretreated patients with R/R FL. Future phase III randomized controlled studies are warranted to establish these findings.
Mosunetuzumab monotherapy3
This multicenter phase II trial (NCT02500407) evaluated the safety and efficacy of mosunetuzumab monotherapy, with step-up dosing in Cycle 1, in heavily pretreated patients (≥2 lines of prior therapy) with R/R FL. The primary endpoint was CRR determined by IRC and key secondary endpoints included PFS and OS.
- At a median follow-up of 28.3 months, 90 patients were included with a median age of 63 years; 61% of patients were male.
- CRR was achieved by 60% of patients, of which 54% achieved CR by end of treatment.
- 24-month duration of CR was achieved by 63% of patients with an ORR of 80%.
- In patients achieving CR, median PFS and OS was not reached; 24-month PFS and OS was 77% and 100%, respectively.
- The median time to first CR was 3 months and there was no association between timing of first CR and DoR.
- Grade ≥2 CRS was reported in 21% of patients and was more frequent in patients with bone marrow disease burden versus those without (33.3% vs 13.8%).
- The median duration of CRS was 3 days and all CRS events were resolved.
Presenter’s conclusions
Fixed-duration mosunetuzumab demonstrated a high CRR at end of treatment in heavily pretreated patients with R/R FL. Responses were sustained, with a high proportion of patients remaining progression free at 2 years. CRS events were low grade and occurred more often in patients with bone marrow metabolic disease burden.
Epcoritamab with rituximab plus lenalidomide4
EPCORE NHL-2 (NCT04663347) is an ongoing phase Ib/II open-label trial. This pooled analysis comprised treatment arms 2a and 2b, including patients with R/R FL who received subcutaneous epcoritamab (48 mg), with step-up dosing, and rituximab (375 mg/m2) plus lenalidomide (20 mg daily) for 12 cycles. The primary endpoints were safety and antitumor activity.
- Overall, 111 patients were treated with a median age of 65 years; 50% were male, 60% had Stage IV disease, 58% had FLIPI score of 3–5, and the median number of prior lines of therapy was one.
- In 104 evaluable patients, ORR was 98%, of which 87% were complete metabolic responses (CMRs).
- There was no difference of antitumor activity in patients who had progression of disease within two years (POD24) versus no POD24; however, CMR was higher in non-POD24 versus POD24 (94% vs 75%).
- Grade ≥3 TEAEs were reported in 76% of patients, 41% of which were related to epcoritamab.
- The most common Grade ≥3 TEAEs were neutropenia (49%) and COVID-19 (17%); immune effector cell-associated neurotoxicity syndrome occurred in 2% and Grade 1–2 CRS occurred in 46% of patients, all of which were resolved.
- At medium follow-up of 11.4 months, 73% of patients were still receiving treatment, with 12% of discontinuations due to COVID-19 and other AEs.
Presenter’s conclusions
Epcoritamab with rituximab plus lenalidomide demonstrated high overall responses and CMRs, regardless of POD24 status. The safety profile was manageable and consistent with previous reports. Other ongoing trials such as EPCORE FL-1 (NCT05409066) are further evaluating epcoritamab subcutaneous combination regimens.
Lisocabtagene maraleucel5
TRANSCEND (NCT04245839) is an open-label, multicenter phase II trial. This primary analysis is focused on assessing the efficacy of lisocabtagene maraleucel (liso-cel) in patients with R/R FL who had received ≥2 prior therapies (3L + FL cohort) and safety across the whole R/R FL cohort (2L + FL). The primary endpoint was ORR determined by IRC, using Lugano 2014 criteria, and key secondary endpoints were CRR, DoR, PFS, and safety.
- Overall, 101 patients were included in the efficacy analysis with a median age of 62 years; 62% of patients were male, 57% had a high-risk FLIPI score, 31% had received prior hematologic stem cell transplant, and 41% had received bridging therapy.
- The primary endpoint was met in the 3L + FL cohort, with an ORR of 97% (p < 0.0001) and a CRR of 94% (p < 0.001); ORR remained consistently high across all subgroups.
- At a median follow-up of 16.6 and 17.5 months, the median DoR and PFS was not reached, respectively.
- The most common Grade 3 TEAEs (≥10%) were neutropenia (58%), lymphopenia (13%), leukopenia (12%), thrombocytopenia (10%), and anemia (10%); serious TEAEs were reported in 25% of patients.
- CRS and neurological events were reported in 58% and 15% of patients, respectively.
- Grade 3 CRS and neurological events occurred in 1% and 2% of patients, respectively.
- There were 12 deaths after liso-cel infusion, of which two were considered related to liso-cel per investigator.
Presenter’s conclusions
Liso-cel achieved deep and durable remissions in patients with R/R FL after three lines of therapy and had a manageable safety profile, with no new safety signals identified. These findings suggest liso-cel as a potential new treatment option for patients with R/R FL.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

