All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Novel therapies in LBCL: Key updates from ICML
Survival outcomes are poor for patients with relapsed/refractory (R/R) large B-cell lymphoma (LBCL). Here, we summarize four key studies on novel therapies for the treatment of LBCL presented at the 17th International Conference on Malignant Lymphoma; Weston presented an overall survival (OS) analysis of axicabtagene ciloleucel (axi-cel) in R/R LBCL,1 Thieblemont presented follow-up data of epcoritamab monotherapy in R/R LBCL,2 Dickinson presented extended follow-up data from the trial of glofitamab monotherapy,3 and Houot presented data on the efficacy of axi-cel as a in second-line therapy in patients ineligible for transplant.4
Axi-cel versus standard of care1
ZUMA-7 (NCT03391466) is global, randomized phase III trial evaluating axi-cel with standard of care (SOC), which consisted of platinum-based chemotherapy followed by high-dose therapy and autologous stem cell transplant (HDT-ASCT) or off protocol additional treatment. The primary endpoint was event-free survival (EFS) and secondary endpoints included OS and progression free survival (PFS).
- Overall, 359 patients were enrolled with a median age of 59; baseline characteristics were generally well balanced between treatment arms, 74% were primary refractory, and 30% of patients were ≥65 years.
- After a median follow-up of 47.2 months, axi-cel significantly improved OS compared with SOC (hazard ratio [HR], 0.726; 95% confidence interval [CI], 0.540-0.977; p = 0.0168); median OS for axi-cel was not reached (NR) and median OS for SOC was 31 months.
- After 4 years, OS rates in axi-cel vs SOC were 54.6% vs 46%, respectively, demonstrating a 27.4% reduction in risk of death with axi-cel.
- The most common Grade ≥3 adverse events (AEs) reported in all patients were cytopenia, cytokine release syndrome (CRS), neurologic events and infections (Figure 1).
The details of the overall trial results have been recently published on the Lymphoma Hub.
Figure 1. Grade ≥3 AEs of interest in patients who received axi-cel versus SOC*
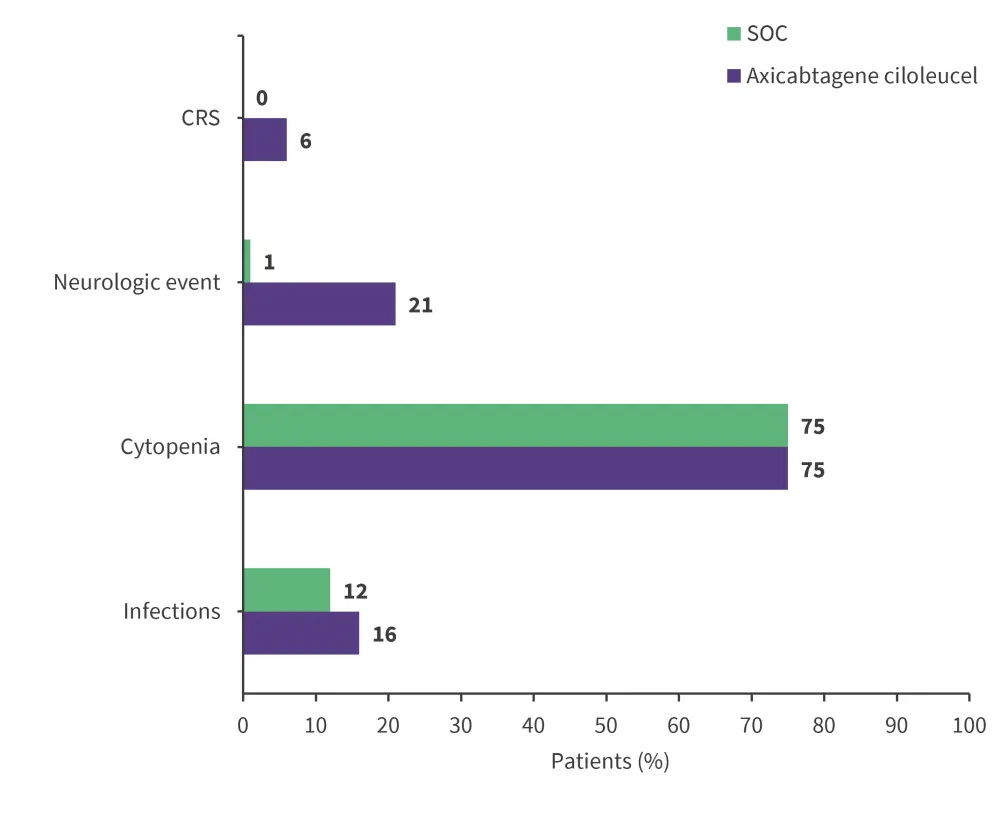
CRS, cytokine release syndrome; SOC, standard of care.
*Adapted from Westin.1
Presenter’s conclusions
Second-line treatment with axi-cel improves OS compared with SOC in patients R/R LBCL, reducing risk of death by 27.4%; despite 57% of patients in SOC arm receiving subsequent cellular immunotherapy. This suggests that the chemotherapy first, CAR T-cell later approach is an inferior option for second-line treatment of these patients. The long-term follow-up OS data are consistent with curative therapy.
Epcoritamab:long term follow-up from the EPCORE NHL-1 trial2
EPCORE NHL-1 (NCT03625037) is a global, multi-center phase I/II trial evaluating epcoritamab in patients with R/R LBCL, previously reported by the Lymphoma Hub. Within the LBCL cohort analyzed, 139 patients had diffuse LBCL (DLBCL), nine had high-grade BCL, four had primary mediastinal BCL, and five had follicular lymphoma Grade 3B. The primary endpoint was overall response rate (ORR) as assessed by independent review committee; key secondary endpoints included duration of response (DoR), time until treatment relapse (TTR), PFS, OS, complete response (CR), and safety.
- A total of 157 patients were enrolled with a median age of 64 years; 70% had received ≥3 previous lines of therapy and 39% had prior chimeric antigen receptor T-cell (CAR T) therapy.
- OR was reached in 63% of patients with LBCL and 39% achieved a complete response; in the subgroup of patients with DLBCL, responses occurred early with a median time to complete response of 2.7 months.
- Median DoR for CR in patients with LBCL was 20.8 months (95% CI, 15.8–NR) with 95% remaining in complete remission after 11 months; median OS was 18.5 months (95% CI, 11.7–NR) in patients with LBCL and 19.4 months (95% CI, 11.7–NR) in patients with DLBCL.
- The most common treatment-emergent AEs (TEAEs) of any grade were CRS, neutropenia, pyrexia, fatigue, nausea, and diarrhea (Figure 2); CRS was largely low-grade and predictable.
- Fatal TEAEs occurred in 15 patients (eight due to COVID-19), with two AEs considered related to therapy: COVID-19 and immune effector cell-associated neurotoxicity syndrome.
Figure 2. Common TEAEs of any grade during treatment with epcoritimab*
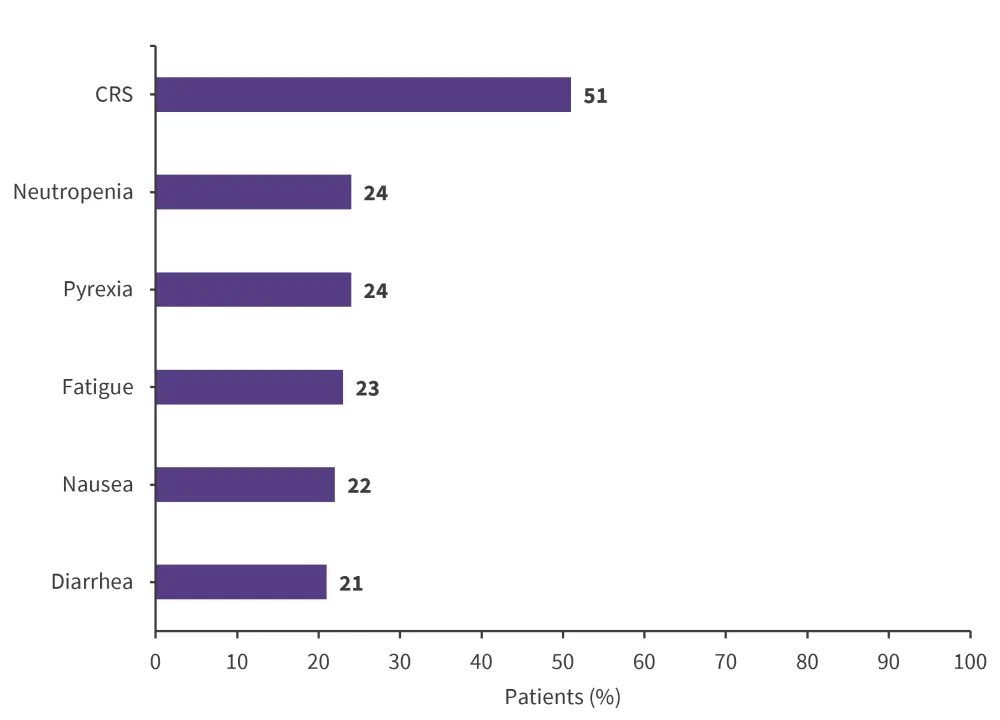
CRS, cytokine release syndrome.
*Adapted from Thieblemont.2
Presenter’s conclusions
Subcutaneous epcoritamab is the first single-agent therapy to show durable complete responses in patients with R/R LBCL. Patients who achieve a complete response have favorable long-term outcomes, with a median OS that was not reached over the course of the study. Safety was manageable, with no new safety signals reported. Thieblemont concluded that this data, along with the recent Food and Drug Administration (FDA) approval of subcutaneous epcoritamab, demonstrates the potential of this treatment for patients with R/R LBCL.
Glofitamab monotherapy3
This is a fixed, multicenter, dose-escalation phase II trial (NCT03075696) designed to assess the efficacy and safety of glofitamab monotherapy in patients with R/R LBCL. The Lymphoma Hub previously reported a visual abstract on the key safety and efficacy data from this trial. The primary endpoint was CR rate and key secondary endpoints included ORR, DoR, PFS, and OS.
- The intent-to-treat population included 155 patients with a median age of 66 years; 65% were male. In addition, 59% were refractory to first therapy, 85% to last prior therapy, and 88% to prior CAR T-cell therapy.
- Out of the 40% of patients who reached CR in the initial analysis, 67% remained in CR at 18 months of follow-up; median duration of CR was 26.9 months (95% CI, 18.4–NR).
- PFS and OS after 18 months, in patients who were in CR by the third cycle of treatment with glofitamab, was 72% and 80%, respectively; PFS and OS at end of treatment was 80% and 92%, respectively.
- Most patients did not experience any new AEs since the previous analysis. The most common AE was CRS, occurring in 64% of patients; 44% of AEs recorded were Grade ≥3 and related to glofitamab treatment. There were no glofitamab-related deaths.
Presenter’s conclusions
This extended follow-up continues to demonstrate that glofitamab treatment results in durable responses, with 40% achieving CR and an estimated 67% of patients reaching CR lasting 18 months. Since the previous analysis, most patients did not experience new AEs. These data and the recent FDA approval, demonstrate that glofitamab as a fixed-duration treatment has the potential to provide long-term benefits to patients with DLBCL.
Axi-cel after first-line treatment4
ALYCANTE (NCT04531046) is an open-label, multicenter phase II trial investigating axi-cel as a second-line therapy in patients with R/R DLBCL who are ineligible for stem-cell transplantation. The primary endpoint was complete metabolic response at 3 months after axi-cel infusion without additional cancer therapy. Key secondary endpoints included EFS, PFS, OS, and safety.
- In total, 62 patients were enrolled across 20 centers in France with a median age of 70; 75.8% were male, 54.8% were refractory to first line therapy, 83.9% received bridging therapy, and 63% did not respond to therapy.
- The study met its primary endpoint, with 71% achieving CR after 3 months; the best objective response was 90.3%, with best CR achieved by 79% of patients. A total of 59.7% patients remained in CR at 6 months post infusion.
- Median EFS from leukapheresis was 12.3 months. Median PFS and OS from infusion was 11.8 months and not reached, respectively.
- Grade 3–4 CRS was reported in 8.1% and Grade 3–4 immune effector cell-associated neurotoxicity syndrome was reported in 14.5%
- At data cutoff, 12 patients had died; five died from lymphoma; six had fatal AEs, such as infection 2 months post infusion; and one death was from an unknown cause.
Presenter’s conclusions
ALYCANTE is the first study to assess safety and efficacy of axi-cel as a second-line therapy in patients with aggressive B-cell lymphoma who were ineligible for allogeneic stem cell transplant. The study met its primary endpoint and axi-cel demonstrated efficacy and an acceptable safety profile in this population, complementing results from the ZUMA-7 trial.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Michael Dickinson
Michael Dickinson Jason Westin
Jason Westin Catherine Thieblemont
Catherine Thieblemont Roch Houot
Roch Houot
