All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Treatment landscape for R/R Waldenstrom's macroglobulinemia
Do you know... Which of the following is a feasible treatment option for patients with Waldenstrom’s macroglobulinemia who experience early relapse (<6–12 months) following first line rituximab-chemotherapy?
Waldenstrom’s macroglobulinemia (WM) is a unique and rare type of non-Hodgkin lymphoma which primarily affects the elderly, with a median age at diagnosis of 70 years. WM presents with a wide variety of clinical sequelae and affects multiple organs, this is due to infiltrated lymphoplasmacytic cells and the immunological properties of the monoclonal IgM protein; the common and uncommon complications of WM have been previously reported on the Lymphoma Hub.1
Patients with WM will eventually experience disease progression following initial treatment; however, many treatment options are in development for this patient subset. Further elucidation of the biology of WM, including identification of the first (myeloid differentiation primary response 88 gene [MYD88]) and second (C-X-C chemokine receptor type 4 [CXCR4]) most prevalent mutations, has played a role in treatment advancements and selection. Further to this, many other disease- and patient-related factors can influence treatment choice in the relapsed/refractory (R/R) setting.
Here, we provide an overview of the current treatment options for R/R WM and associated factors that influence treatment indications in this setting.1
Current treatment options for R/R WM
Current treatment options for R/R WM include immunochemotherapy, proteasome inhibitors, and Bruton’s tyrosine kinase inhibitors (BTKis).
Chemoimmunotherapy options
Dexamethasone, rituximab, and cyclophosphamide is a chemoimmunotherapy option that has proven to be highly efficacious in the relapsed setting.1,2After six complete cycles in 71% of patients with R/R WM, the overall response rate (ORR) was 87%; this included a 4% very good partial response (VGPR), 64% partial response (PR), and 19% minor response rate.1
Bendamustine combined with rituximab (benda-R) is another immunochemotherapy option in R/R WM.1,2 In one retrospective analysis involving 111 R/R WM patients, benda-R achieved a major response rate (MMR) of 74%. In another retrospective study of benda-R in 71 patients with R/R WM, an ORR and MRR of 80.2% and 74.6%, respectively, was achieved; neutropenia (13%) was the most reported Grade 3/4 adverse event (AE).1
Proteasome inhibitor (PI)-based therapy1
Proteasome inhibitors (PI) are an efficacious treatment option for R/R WM.2 In a WMCTG trial, bortezomib monotherapy has resulted in an ORR of 85%, a median time to treatment response of 1.4 months, and median time to progression of 7.9 months in patients with R/R WM; Grade 3/4 peripheral neuropathy was the most commonly reported AE. Another study of bortezomib monotherapy achieved an ORR of 78%, median progression-free survival (PFS) of 16.3 months, and 74% of patients experiencing neuropathy or worsening of existing neuropathy.1
A phase 2 trial of bortezomib combined with rituximab in patients with R/R WM (n = 37) demonstrated an ORR of 81% and a median PFS of 15.6 months; the most commonly reported Grade 1 and 2 AEs were anemia (81%), fatigue (68%), neuropathy (41%), and diarrhea (37%). Of note, the occurrence of peripheral neuropathy is of concern in the use of bortezomib.1
The oral PI ixazomib combined with rituximab and dexamethasone attained an ORR of 71%, with PFS and overall survival (OS) rates of 56% and 88%, respectively, in a phase 1/2 trial of 59 patients with R/R WM. Ixazomib was well-tolerated and had a manageable toxicity profile, with no Grade 3 toxicities reported and most neurotoxicities being Grade 1 or 2.1
BTK inhibitors
Ibrutinib, an oral BTKi, was the first to receive U.S. Food and Drug Administration (FDA) approval for the treatment of patients with WM in 2015; this approval was based on its highly efficacious responses in patients with R/R WM.1,2 A prospective study in 63 patients demonstrated an ORR and MRR of 90.5% and 73%, respectively, with response rates seen to correlate with MYD88/CXCR4 status. The 2-year PFS and OS rates were 69.1% and 95.2%, respectively, and the most common reported Grade ≥2 AEs were neutropenia (22%), thrombocytopenia (14%), postprocedural bleeding (3%) and atrial fibrillation (5%).1
In the analysis of patients with rituximab-refractory WM (n = 31), ibrutinib achieved an ORR and MRR of 90 and 71%, respectively, after a median follow-up of 18 months.1
The INNOVATE trial (NCT02165397; N = 150), in which 55% of patients had previously been treated for WM, demonstrated the following results in ibrutinib-rituximab versus placebo-rituximab cohorts: a higher MRR (72% vs 32%); higher Grade 3 toxicities (12% vs 1%); higher rate of hypertension (13% vs 4%); and higher 30-month-PFS (82% vs 28%).1
Zanubrutinib is another FDA approved BTKi for WM, this approval was based on results of the phase III ASPEN trial (NCT03053440) comparing its efficacy to ibrutinib. The study included 201 patients (R/R WM, n = 164; treatment-naïve, n = 37) and yielded a similar ORR (94 vs 93%) and MRR (77% vs 78%) for the zanubrutinib versus ibrutinib arm, higher VGPR responses in the zanubrutinib arm (28% vs 19%), but comparable PFS rates overall. There was a higher prevalence of atrial fibrillation/flutter in the ibrutinib vs zanubrutinib arm (15% vs 2%), higher frequency of Grade ≥3 hypertension and pneumonia in ibrutinib arm, a similar frequency of infections between the two arms, and a 5% higher incidence of Grade ≥3 neutropenia. Overall, zanubrutinib proved to be the safer option in this setting.1,2
In a prospective phase 2 study of zanubrutinib, which included 53 patients with R/R WM and 24 treatment-naïve patients, ORR was 94% and VGPR/complete response (CR) was 51% in the R/R group. A similar phase II study in 44 R/R WM patients demonstrated an ORR, MRR, and VGPR/CR of 77%, 70%, and 33%, respectively.1
Acalabrutinib has been proven as an effective BTKi in R/R WM. A phase II multicenter study (R/R WM, n = 92; treatment-naïve WM, n=14) reported an ORR and MRR of 93% and 78%, respectively. The most common AEs were headache, diarrhea, dizziness, fatigue, nausea, joint pain, and upper respiratory tract infections; neutropenia (16%) and pneumonia (7%) were the most common Grade 3–4 AEs.1,2
A phase II study which assessed Tirabrutinib in 27 patients with WM (R/R WM, n = 9; treatment-naïve, n = 18) demonstrated an ORR of 94–100%, with rash (44.4%) and neutropenia (25.9%) reported as the most frequently observed AEs. Preliminary results on orelabrutinib, which is currently being investigated in a phase II study, reported an ORR and MRR of 87.2% and 74.5% at a median 10.5 month follow-up and 12-month PFS and OS rates of 88.0% and 92.3%, respectively; 34% of patients had Grade ≥ 3 AEs. To overcome the acquired resistance seen in covalent BTKis, the non-covalent BTKi pirtobrutinib has been investigated, with a phase 1/2 trial demonstrating safety and efficacy even in 26 patients previously treated with covalent BTKis.
Other emerging treatments1
BCL2 inhibitors
BCL2 inhibitors, such as venetoclax, are emerging options for R/R WM. Amongst 31 patients with R/R WM treated with venetoclax in a phase II clinical trial, the ORR and MRR was 87 and 80%, respectively, at median 33-month follow-up of; median PFS was 30 months. Grade ≥3 neutropenia occurred in 45% and Grade ≥2 AEs occurred in 94% of patients.
Anti-CXCR4 monoclonal antibodies
Given the impact of CXCR4 mutational status on clinical outcomes for BTKis in WM, phase I and II trials are currently investigating CXCR4-targeted agents. Ulocuplumab, a CXCR4 antagonist, has been assessed in combination with ibrutinib in a phase 1/2 clinical trial of 13 WM patients (R/R, n = 4 ) results showed an MRR of 100% at median 22.4 months follow and a 2-year PFS of 90%. Mavorixafor, another CXCR4 antagonist, demonstrated an ORR of 100% alongside a decrease in IgM levels when combined with ibrutinib in a phase I study of nine patients with WM; only nine mavorixafor-related AEs were reported, two of which led to drug interruptions.
T-cell therapies
T-cell targeted immune therapies, such as bispecific antibodies, are emerging as a beneficial option in the treatment of WM given its favorable toxicity profile and availability as an off-the-shelf product. A phase I trial of a CD20 × CD3 antibody that included three patients with R/R WM, revealed the most common Grade 3 AEs as anemia, lymphopenia, infections and infestations, and neutropenia.
Treatment decision making for R/R WM1
It is recommended that treatment should be initiated in symptomatic cases of WM. For patients with R/R WM, various factors should be considered when selecting the most optimal and personalized therapeutic option including duration (fixed duration vs ongoing), toxicity, duration of response after last treatment, patient age, comorbidities, patient and physician perceived treatment goals, and mutation profiles.
Mutational status is an increasingly used algorithmic tool for tailoring treatment options in patients with R/R WM. Limited data have shown similar responses between MYD88mut and MYD88wt for patients treated with immunochemotherapy or PIs, revealing no impact of MYD88 mutational status on outcomes of patients with WM for these treatment types.1,2 Similarly, preliminary data have shown no impact of CXCR4 mutational status on patients treated with BCL2 inhibitors—such as venetoclax.2
On the other hand, mutational analysis is especially important for BTKis and probably other novel targeted therapies, such as anti-CXCR4 antibodies, due to their differential activity based on patient genotypes. For example, there was lower ORR and MRR responses observed in patients with MYD88wt (ORR, 79%; MRR, 57%) versus MYD88L265P (ORR, 94%; MRR, 78%) in a phase II study of acalabrutinib; treatment outcomes by mutational status for other BTKis are detailed in Table 1. An example of a genomic-based algorithm for R/R WM was previously reported on the Lymphoma Hub.
Table 1. Response rates by mutational status in selected R/R WM trials on BTKis*
|
BTKi, Bruton’s tyrosine kinase inhibitor; MRR, major response rate; mut, mutated; NR, not reported; ORR, overall response rate; R/R relapsed/refractory; TN, treatment-naïve; WM, Waldenstrom’s macroglobulinemia; wt, wild-type. |
|||||
|
Response, % (unless |
MYD88mut/CXCR4wt |
MYD88mut/CXCR4mut |
MYD88wt/CXCR4wt |
||
|---|---|---|---|---|---|
|
Ibrutinib monotherapy in patients with R/R WM (n = 63) |
|||||
|
ORR |
100 |
85.7 |
71.4 |
||
|
MRR |
91.2 |
61.9 |
28.6 |
||
|
Ibrutinib in patients with rituximab-refractory WM (n = 31) |
|||||
|
ORR |
88 |
100 |
— |
||
|
MRR |
82 |
71 |
— |
||
|
Zanabrutinib in patients with R/R WM (n = 77 [TN, n = 24; R/R, n = 53]) |
|||||
|
ORR |
97 |
100 |
100 |
||
|
MRR |
87 |
91 |
63 |
||
|
Ibrutinib + rituximab in TN and R/R WM (N = 150, [iNNOVATE NCT02165397]) |
|||||
|
ORR |
94 |
100 |
81 |
||
|
MRR |
NR |
— |
— |
||
For patients with early relapse (<6–12 months) after first-line rituximab-chemotherapy, BTKis are the preferred regimen and bortezomib is an alternative option. Stem cell transplantation is a viable option for younger and fit patients who experience both early relapse to rituximab-containing immunochemotherapy and resistance/tolerance to BTKis. For patients with late relapse (±3 years) after immunochemotherapy, an alternative immunochemotherapy, prior effective regimen, a PI, or BTKi monotherapy or ibrutinib/rituximab are valid options in this case (Figure 1).4 In general, participation of patients in clinical trials is encouraged in the relapsed/refractory setting.
Figure 1. Treatment approach for early vs late relapse*
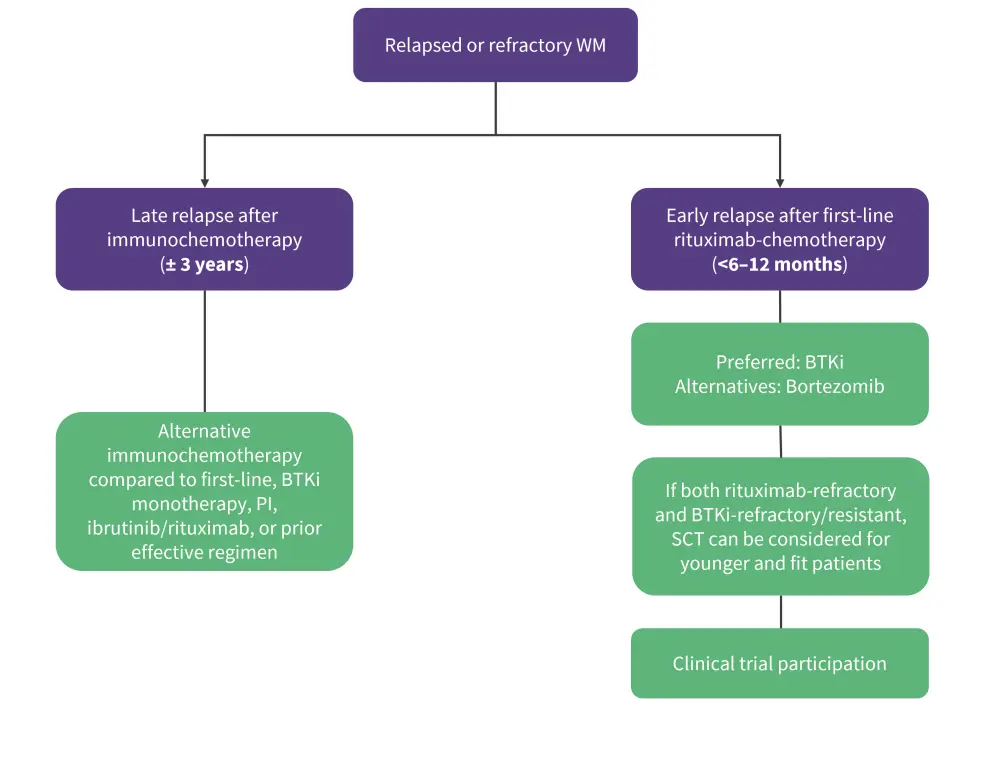
BTKi, Bruton’s tyrosine kinase inhibitors; PI, proteasome inhibitor; SCT, stem cell transplantation; WM, Waldenstrom’s macrobulinemia
*Adapted from Amaador, et al.1 and Grunenberg, et al.4
Conclusion
Although there are many treatment options available for R/R WM, including immunochemotherapies, BTKis, and PIs there is no consensus treatment approach given the paucity of randomized clinical trials. Treatment selections in this setting should be individualized by considering several factors including age, duration of treatment, toxicities, duration of response after last treatment, comorbidities, and MYD88 and CXCR4 mutational status.
With the evolving molecular landscape of WM, we anticipate the introduction of further novel treatments in R/R WM. Future clinical trials should focus on patient preference including nontoxic treatment and fixed-duration therapeutic options.
‘The International Waldenstrom's Macroglobulinemia Foundation (IWMF) and the Lymphoma Hub are working in collaboration for patients with Waldenstrom's macroglobulinemia. This initiative aims to increase awareness of Waldenstrom's macroglobulinemia among healthcare professionals, patients, caregivers, and the patient advocacy community.
This initiative is funded by Cellectar Biosciences. All content is developed independently by SES in collaboration with an expert steering committee; funders are allowed no direct influence on the content of the hub.’
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content




