All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Do you know... Which of the following therapies is approved in both first-line and relapsed or refractory Hodgkin lymphoma in the EU?
Video series
During the European School of Haematalogy (ESH) 4th How to Diagnose and Treat Lymphoma Conference, the Lymphoma Hub held a symposium on November 2, 2024, titled, Treating classic Hodgkin lymphoma (cHL): Spotlight on targeted therapies. Here, we share a presentation by Anna LaCasce, Harvard Medical School, Boston, US, discussing the current landscape of targeted therapies for cHL.
LaCasce begins by highlighting key molecular targets for the treatment of cHL, such as CD30 and PD-1 (Figure 1).1 She discusses approved therapies targeting these pathways, including brentuximab vedotin for CD30, and checkpoint inhibitors nivolumab and pembrolizumab for PD-1, along with their clinical indications (Figure 2).
Figure 1. Key cellular targets in the treatment of cHL*
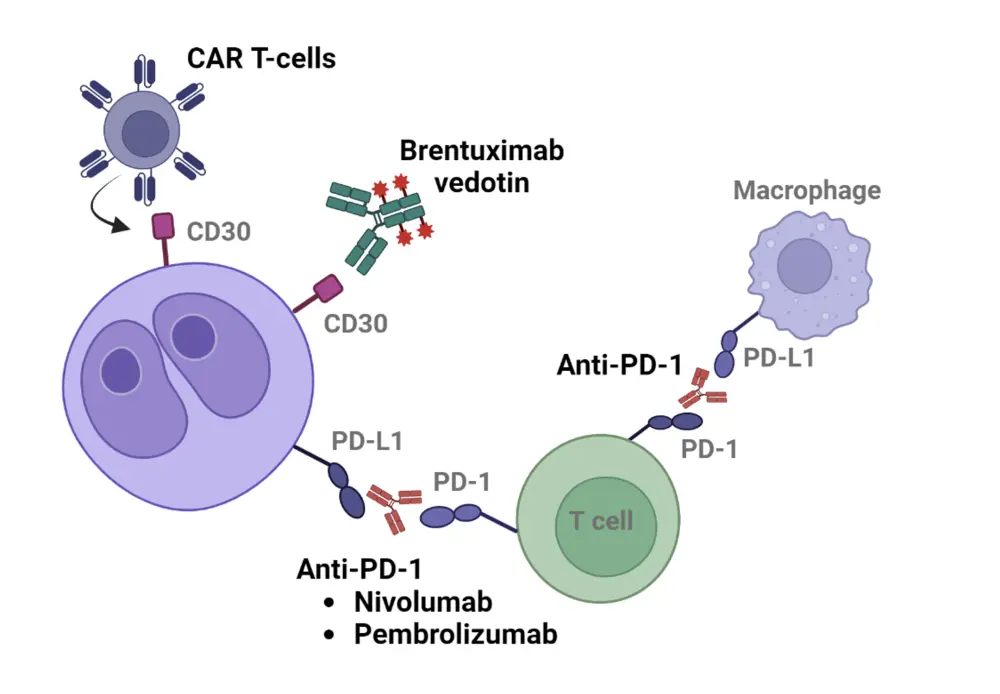
CAR, chimeric antigen receptor; CD, cluster of differentiation; cHL, classic Hodgkin lymphoma; PD-1, programmed death 1.
*Adapted from Wu, et al.1 Created using Biorender.com.
Figure 2. Overview of approved targeted therapies for cHL
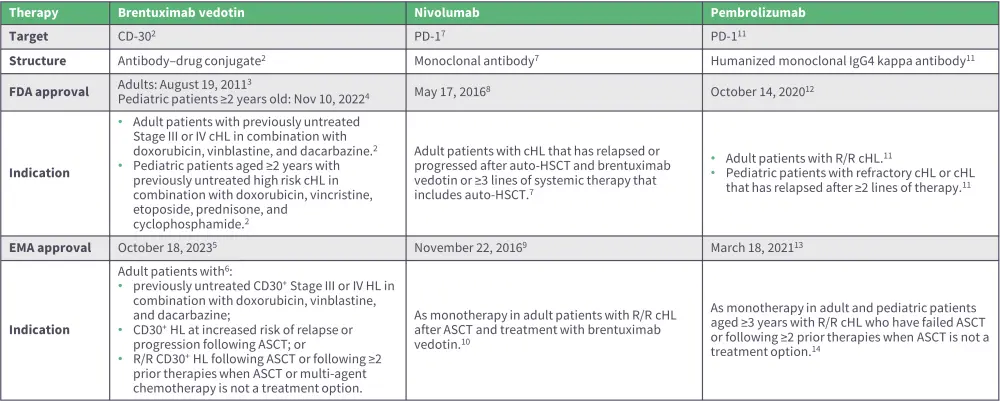
ASCT, autologous stem cell transplant; auto-HSCT, autologous hematopoietic stem cell transplantation; CD, cluster of differentiation; cHL, classic HL; HL, Hodgkin lymphoma; PD-1, programmed death 1; R/R relapsed/refractory.
LaCasce provides an overview of the key clinical trials and their outcomes for approved targeted therapies in cHL:
Brentuximab vedotin (BV)
- The pivotal phase II trial (NCT00848926) of BV in heavily pre-treated patients with cHL showed an overall response rate (ORR) of 75%, with about a third of patients achieving a complete remission (CR). Peripheral sensory neuropathy was the most common adverse event (AE) and tended to improve over time after the completion of therapy.
- The phase III AETHERA trial (NCT01100502) evaluated BV as a maintenance therapy after autologous hematopoietic stem cell transplantation (auto-HSCT) in patients with high-risk relapse disease. The 5-year progression-free survival (PFS) in the BV arm was 59% compared with 41% in the placebo arm, with patients having ≥3 risk factors benefiting the most.
- The phase III ECHELON-1 trial (NCT01712490) evaluated BV + AVD (doxorubicin, vinblastine, and dacarbazine) vs bleomycin + AVD (ABVD) in patients with Stage 3 or 4 cHL. Modified PFS improved by 4% in the BV+AVD arm vs ABVD arm, with this survival benefit sustained over 5 years of follow-up and observed in both PET-positive and -negative patients.
- The phase III AHOD1331 trial (NCT02166463) evaluated BV + standard chemotherapy in pediatric patients with cHL, aged 2–21 years. The 3-year PFS and OS rates with BV were 92.1% and 99.3%, respectively, compared with 82.5 and 98.5% with standard care.
- The phase III HD-21 trial (NCT02661503) evaluated BrECADD (brentuximab vedotin, etoposide, cyclophosphamide, doxorubicin, dacarbazine, and dexamethasone) vs escalated BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone) in patients with newly diagnosed, advanced-stage, PET-2-positive cHL. The 4-year PFS rates in the BrECADD arm were 94.3%, the highest ever reported in clinical trials in patients with newly diagnosed, advanced-stage, PET-2-positive cHL, compared with 90.9% in the escalated BEACOPP.
Nivolumab
- The phase I/II CHECKMATE 039 trial (NCT01592370) showed that nivolumab had a manageable safety profile and an ORR of >80% in patients with previously heavily treated R/R cHL.
- The phase II CHECKMATE 205 trial (NCT02181738) showed that nivolumab exhibited durable responses, particularly in a subset achieving CR, and a manageable safety profile in patients with R/R cHL following auto-HSCT.
- The phase III SWOGS1826 trial (NCT03907488) evaluating nivolumab + AVD vs BV + AVD in newly diagnosed patients with advanced stage cHL showed a 2-year PFS of 92% vs 83%, respectively. The safety profile was more favorable with nivolumab + AVD compared with BV + AVD.
Pembrolizumab
- The phase III KEYNOTE-204 (NCT02684292) compared pembrolizumab and BV in patients with R/R cHL who had relapsed following auto-HSCT or were not eligible for auto-HSCT. The median PFS was 13.2 months with pembrolizumab vs 8.3 months with BV.
- The phase II KEYNOTE-087 trial (NCT02453594) evaluating pembrolizumab monotherapy in patients with R/R cHL showed durable response, particularly in a subset achieving CR.
Your opinion matters
As a result of my participation in this symposium, I commit to staying aware of the latest clinical trial updates and guidelines for treatment sequencing in patients with cHL and to consider using targeted therapies when appropriate.
This independent educational activity was supported by Takeda. All content was developed independently by the faculty in collaboration with SES. The funder was allowed no influence on the content of the symposium.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Ann Lacasce
Ann Lacasce

