All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Do you know... Which of the following statements is true regarding CAR T-cell therapies?
During the 18th International Conference on Malignant Lymphoma (ICML), June 17–21, 2025, Lugano, CH, the Lymphoma Hub held a symposium on June 17, 2025, titled Customizing therapy for mantle cell lymphoma (MCL). Here, we share a presentation by Michael Wang, MD Anderson Cancer Center, Houston, US, discussing current therapies for MCL and their combinations.
Symposium | Current therapies for MCL and their combinations
Symposium | Current therapies for MCL and their combinations
Wang provided an overview of current therapies for MCL, highlighted key clinical data, and offered insights into their clinical application. He also discussed novel therapies and combinations, and potential future directions.
Key points
Bortezomib
- In the phase II PINNACLE trial, single-agent bortezomib was associated with an overall response rate (ORR) of 32% and a median progression-free survival (PFS) of 6.7 months in patients with relapsed/refractory (R/R) MCL.1,2
- In the phase III LYM-3002 trial (NCT00722137), bortezomib in combination with rituximab + cyclophosphamide + doxorubicin + prednisone (VR-CAP) improved ORR (92% vs 89%) and median PFS (24.7 months vs 14.4 months) vs rituximab + cyclophosphamide + doxorubicin + vincristine + prednisone (R-CHOP) in patients with newly diagnosed (ND) MCL.3
Lenalidomide
- Results from the phase II EMERGE (MCL-001) trial (NCT00737529) showed the single-agent activity of lenalidomide in patients with R/R MCL, with an ORR of 28% and a median PFS of 4 months.4
- The phase III SPRINT (MCL-002) trial (NCT00875667) compared single-agent lenalidomide with investigator’s choice in patients with R/R MCL; ORR was 40% vs 11% and median PFS was 8.6 months vs 5.4 months, respectively.5,6
Ibrutinib
- A pooled analysis of 370 patients with R/R MCL from the phase II PCYC-1104 (NCT01236391) and SPARK (NCT01599949) and the phase III RAY (NCT01646021) trials assessed the long-term efficacy of ibrutinib monotherapy.7
- Median follow-up was 9.7 years.
- The ORR and complete response (CR) rates were 69.7% and 27.6%, respectively.
- The median duration of response (DoR), PFS, and overall survival (OS) were 21.8 months, 12.5 months, and 26.7 months, respectively.
- The phase III SHINE trial (NCT01776840) evaluated ibrutinib in combination with bendamustine + rituximab (BR) and rituximab maintenance in 523 patients aged ≥65 years with previously untreated MCL.8
- Median follow-up was 84.7 months.8
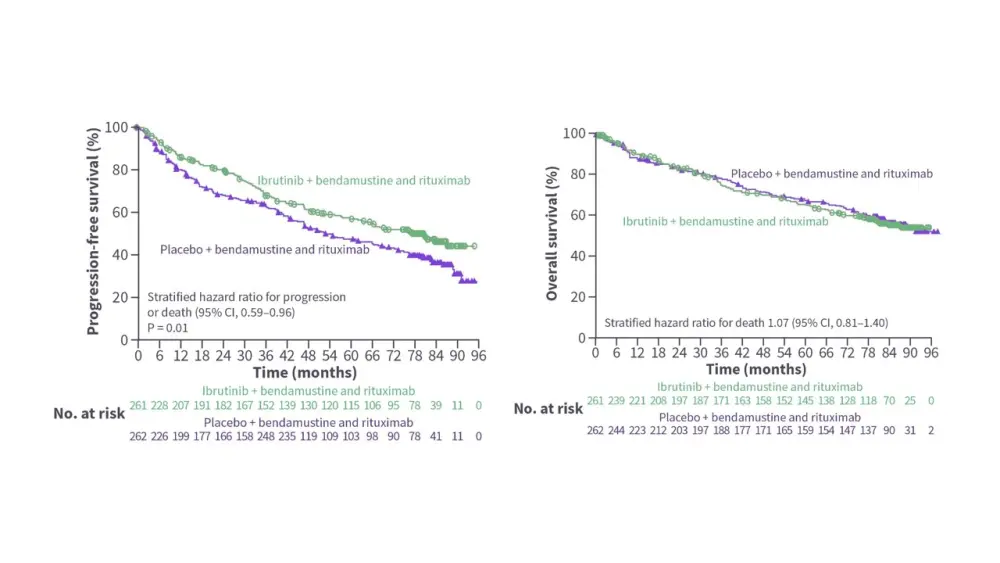
- Ibrutinib + BR improved PFS vs placebo + BR; however, OS was similar between the two groups (Figure 1).8
- The rates of Grade 3/4 atrial fibrillation (3.9% vs 0.8%) and pneumonia (20.1% vs 14.2%) were higher in the ibrutinib + BR group.8
- Based on results from this trial, the US indications for ibrutinib for the treatment of patients with MCL were withdrawn.9
Figure 1. Survival outcomes in the phase III SHINE trial*
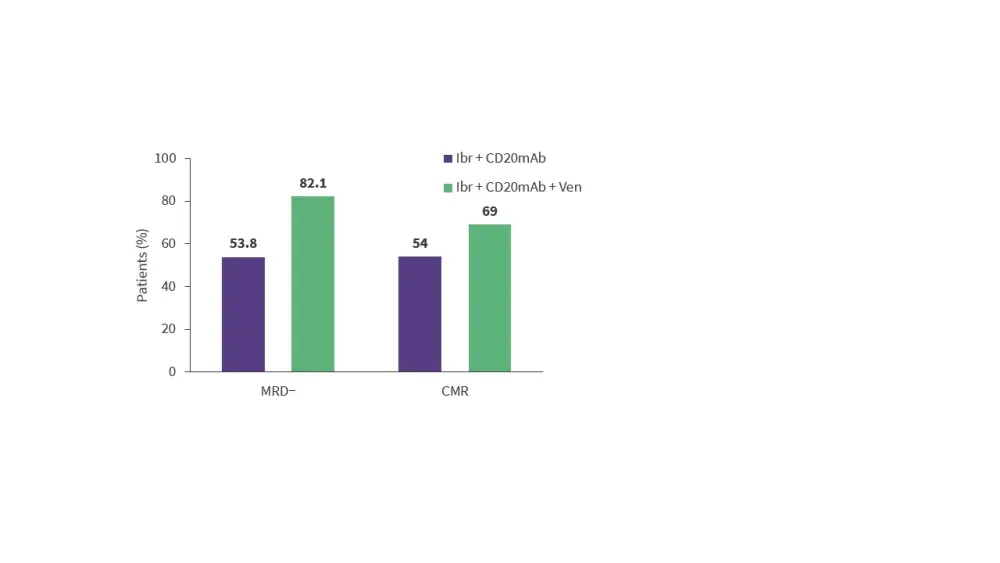
- The ongoing phase II OASIS II trial (NCT04802590) is investigating ibrutinib + CD20 monoclonal antibody (rituximab or obinutuzumab) ± venetoclax in treatment-naïve patients with MCL.
- Results from 102 patients treated with ibrutinib + CD20 monoclonal antibody ± venetoclax showed that this regimen was associated with high measurable residual disease (MRD) negativity rates (Figure 2).
Figure 2. Response rates in the phase II OASIS II trial*
Acalabrutinib
- Results from the phase II ACE-LY-004 trial (NCT02213926) demonstrated high response rates with acalabrutinib in patients with R/R MCL (ORR, 81.5%; CR, 47.6%).11
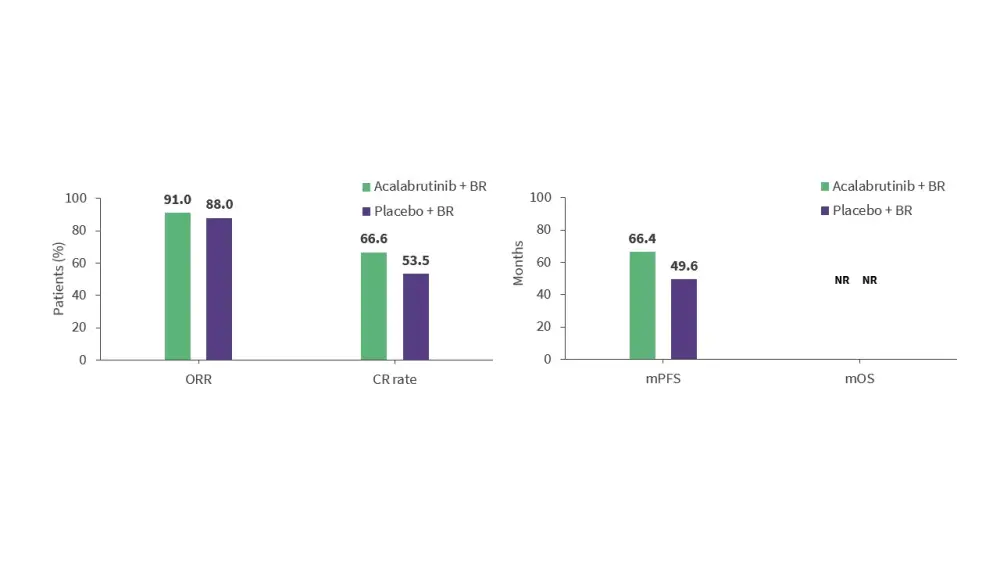
- The phase III ECHO trial (NCT02972840) assessed acalabrutinib in combination with BR and rituximab maintenance in 598 patients aged ≥65 years with previously untreated MCL.12
- Acalabrutinib + BR was associated with high response rates, and improved PFS vs placebo + BR (Figure 3).
Figure 3. Response rates and survival outcomes in the phase II ECHO trial*
- An ongoing phase Ib trial (NCT02717624) is evaluating acalabrutinib in combination with venetoclax + rituximab in treatment-naïve patients with MCL.
- Initial results from 21 patients showed high response rates (ORR, 100%; CR, 74%), and, with a median follow-up of 27.8 months, median PFS and OS have not been reached.13
Zanubrutinib
- Long-term results from a phase II trial (NCT03206970) of zanubrutinib in 86 patients with R/R MCL showed high response rates (ORR, 83.7%; CR, 67.0%).14
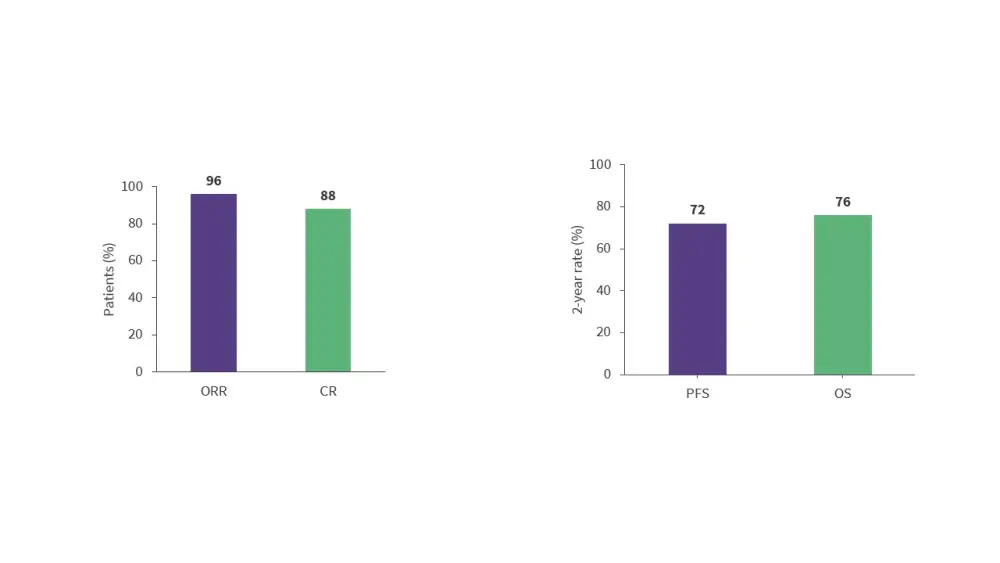
- The ongoing phase II BOVen trial (NCT03824483) is assessing zanubrutinib, obinutuzumab, and venetoclax (BOVen) in treatment-naïve patients with TP53-mutated MCL.15
- With a median follow-up of 28.2 months, the BOVen regimen was associated with high response rates and 2-year survival rates (Figure 4).
Figure 4. Response rates and 2-year survival rates with the BOVen regimen*
Pirtobrutinib
- Results from the phase I/II BRUIN trial (NCT03740529) demonstrated the efficacy of pirtobrutinib, a non-covalent BTKi, in 152 patients with R/R MCL who were previously treated with covalent BTKi (Figure 5).16
- This trial included 14 patients who were BTKi-naïve; the ORR and CR rates were 85.7% and 42.9%, respectively, in these patients.16
Figure 5. Response rates and survival outcomes in the phase I/II BRUIN trial*
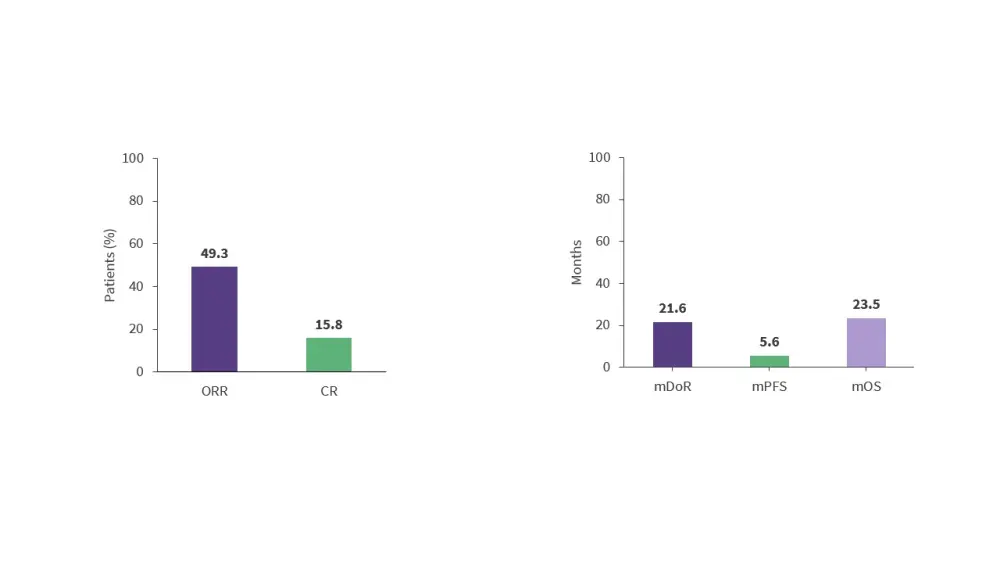
- Extended (≥12 months) drug exposure data from the BRUIN trial suggest that pirtobrutinib continues to demonstrate a favorable safety profile without evidence of new or worsening toxicity signals.17
- Several trials assessing pirtobrutinib-based combination therapies in patients with MCL are currently recruiting:
- A phase II trial (NCT05529069) of pirtobrutinib + venetoclax in patients with R/R MCL.18
- The phase II GOlDiLOX trial (NCT05833763) of glofitamab + pirtobrutinib in patients with R/R MCL.19
- A phase II trial (NCT06263491) of pirtobrutinib + rituximab in patients with previously untreated low-/intermediate-risk MCL.20
- The phase II GATE1 trial (NCT06522386) of pirtobrutinib + rituximab + venetoclax in patients with previously untreated MCL.21
- The phase III BRUIN MCL-321 trial (NCT04662255) will evaluate pirtobrutinib vs covalent BTKi (ibrutinib, acalbrutinib, or zanubrutinib) in patients with previously treated BTKi-naïve MCL.22
Nemtabrutinib
- The ongoing phase II BELLWAVE-003 trial (NCT04728893) is assessing nemtabrutinib, a non-covalent BTKi, monotherapy after covalent BTKi failure in patients with hematologic malignancies (N ≈ 490), including a cohort of patients with MCL.23
- The ongoing phase II waveLINE-006 study (NCT05458297) is evaluating zilovertamab vedotin in combination with nemtabrutinib in patients with R/R aggressive and indolent B-cell malignancies, including a cohort of patients with R/R MCL.24
- Initial results from the R/R MCL cohort (n = 28) showed an ORR and CR rate of 64% and 32%, respectively.24
BTK degraders
- Several BTK degraders that have shown preliminary activity in patients with MCL are currently being investigated in clinical trials:
- The phase I NX-2127-001 trial (NCT04830137) and the phase I NX-5948-301 trial (NCT05131022) are evaluating NX-2127 and bexobrutideg (NX‑5948) in patients with R/R B-cell malignancies, including MCL.25,26
- The phase I/II CaDAnCe-101 trial (NCT05006716) is investigating BGB-16673 in patients with R/R B-cell malignancies, including MCL.27
Brexucabtagene autoleucel
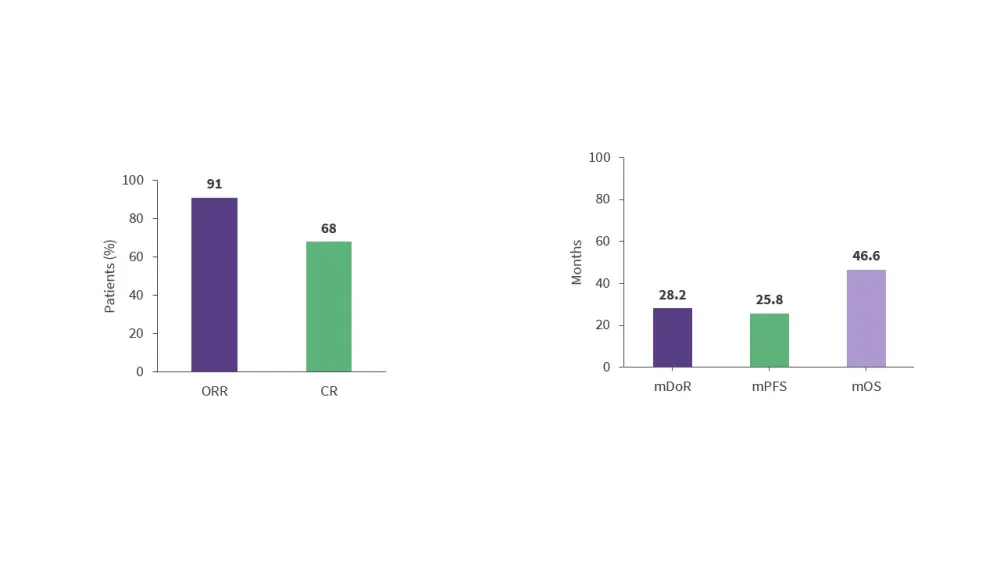
- 3-year follow-up results from the phase II ZUMA-2 trial (NCT02601313) showed that brexucabtagene autoleucel (brexu-cel) is associated with high response rates in patients with R/R MCL (Figure 6).28
- Cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) occurred in 91% and 63% of patients, respectively.28
Figure 6. Response rates and survival outcomes in the phase II ZUMA-2 trial*
Lisocabtagene maraleucel
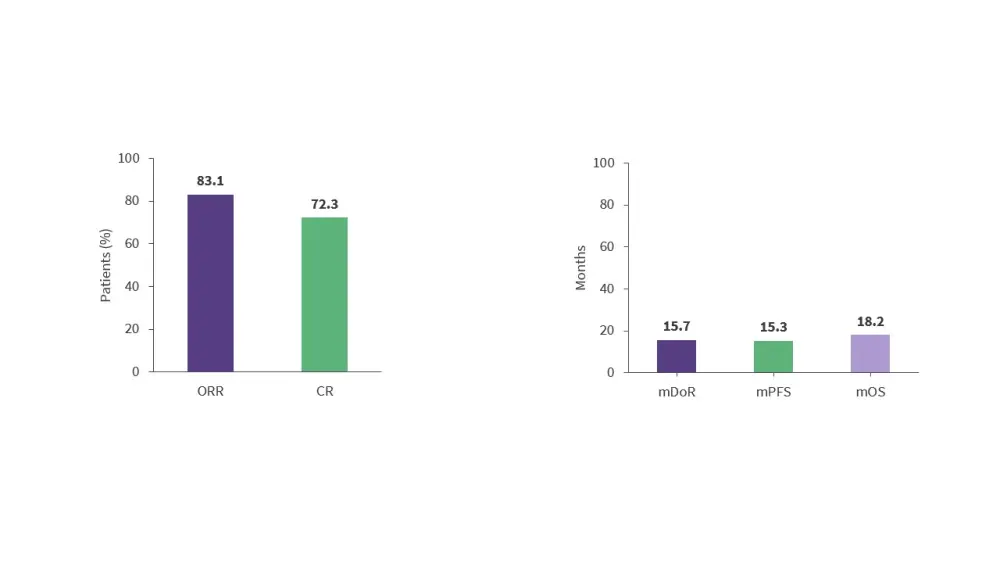
- Results from the phase I TRANSCEND NHL 001 trial (NCT02631044) demonstrated the efficacy of lisocabtagene maraleucel (liso-cel) in patients with R/R MCL (Figure 7).29
- Liso-cel demonstrated a favorable safety profile, which may allow for outpatient treatment and combination therapy with other targeted immunotherapies.29
Figure 7. Response rates and survival outcomes in the phase I TRANSCEND NHL 001 trial*
Wang concludes by highlighting the need for novel therapies and combinations, particularly in patients who are not able to receive chimeric antigen receptor (CAR) T-cell therapies.
This educational resource is independently supported by Eli Lilly and Company. All content was developed by SES in collaboration with an expert steering committee. Funders were allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Michael Wang
Michael Wang
.webp&w=3840&q=75)
.webp&w=3840&q=75)
.webp&w=3840&q=75)